Amplification-effect-free continuous flow synthesis process of tert-butyl peroxyneodecanoate
A technology for tert-butyl peroxyneodecanoate and tert-butanol peroxide, which is applied in the field of chemistry, can solve the problems of long total reaction time, low esterification reaction temperature, decreased production efficiency, etc., and achieves stability and reliability. Good, solve the amplification effect, save the effect of plant land
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
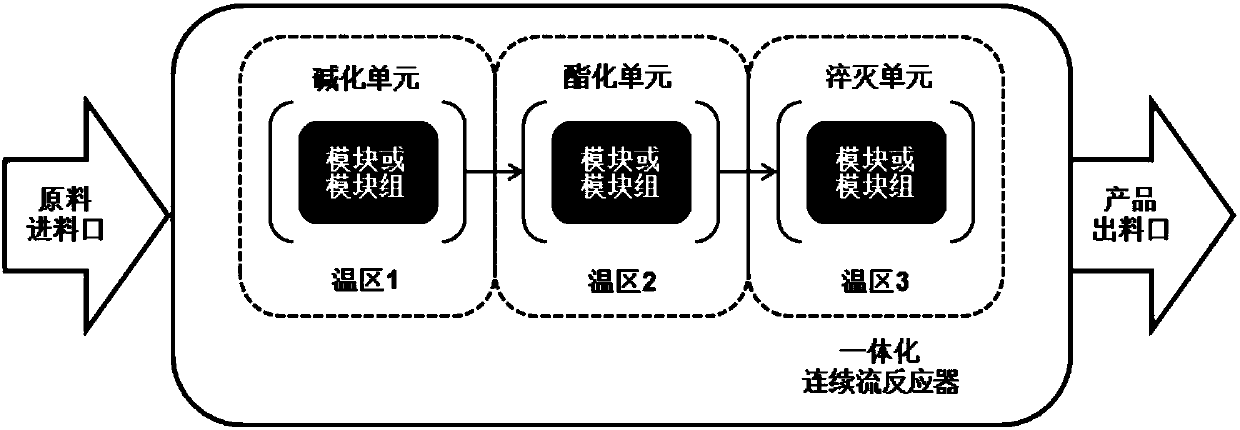
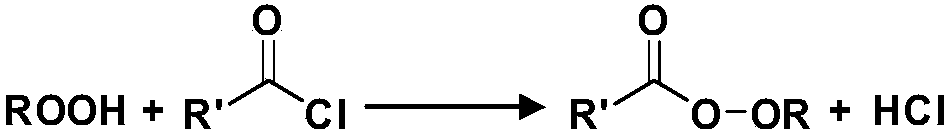
[0098] Such as figure 1 and figure 2As shown, raw material 1 (tert-butanol peroxide) and raw material 2 (potassium hydroxide aqueous solution) are transported into temperature zone 1 with a constant flow pump, and flow through temperature zone 1 to react to generate intermediate tert-butyl potassium peroxide . The raw material 3 (neodecanoyl chloride) is transported into the temperature zone 2 by using a constant flow pump, and the reaction is complete after flowing through the temperature zone 2. The reaction solution flowing out of temperature zone 2 enters temperature zone 3 to cool down to quench the reaction, and collect the reaction mother liquor. The mother liquor is separated, washed, etc., and tert-butyl peroxyneodecanoate can be obtained. The reaction parameters and results are as follows:
[0099] Table 1: Raw material flow rate, warm zone temperature, reaction time, content and yield
[0100]
Embodiment 2-19
[0102] Adopt the operating method of embodiment 1, investigated the reaction time, content and yield of preparing tert-butyl peroxyneodecanoate under different reaction parameters, each parameter condition and result are as shown in table 2 and 3 below.
[0103] Table 2: Raw material concentration and flow rate of Examples 2-19
[0104]
[0105] Table 3: Temperature, reaction time, content and yield of each temperature zone of embodiment 2-19
[0106]
[0107]
[0108] a The concentration of tert-butanol peroxide used in the actual synthesis will have a mass concentration deviation of ± 3 percentage points from the concentration listed in the table.
[0109] b The lye concentration used in the actual synthesis and the concentration listed in the table will have a mass concentration deviation of ± 3 percentage points.
[0110] c There will be a deviation of ±5°C between the temperature of the temperature zone in the actual synthesis and the temperature listed in the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com