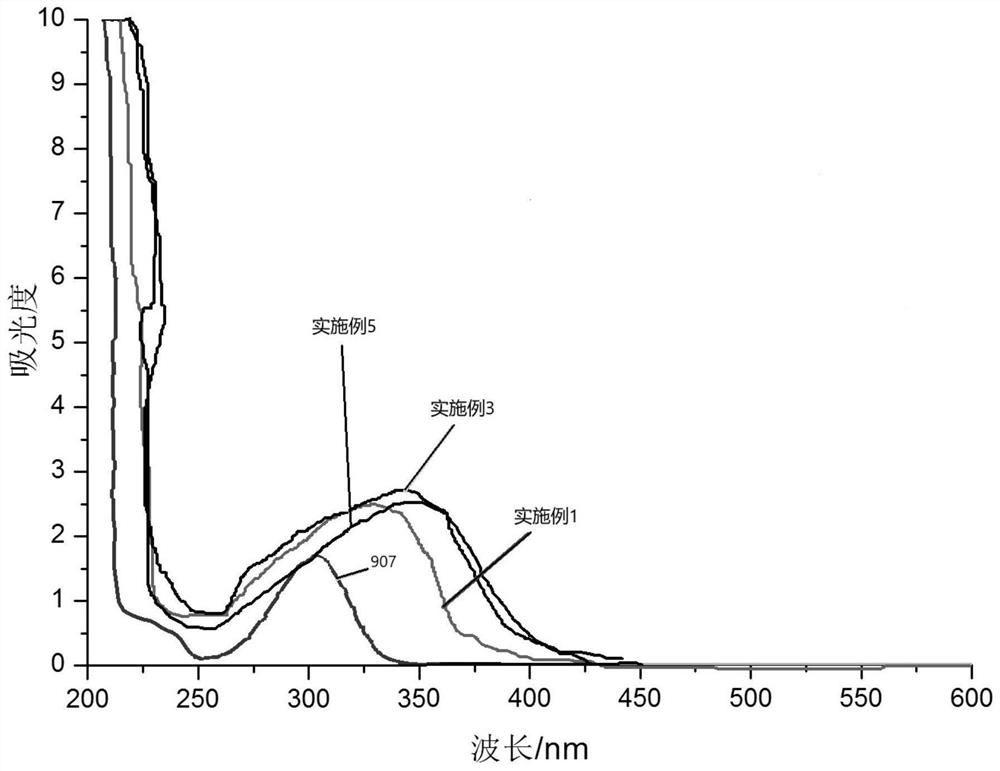
Phenazine derivative-series photoinitiator as well as preparation method and application thereof
A technology of photoinitiators and derivatives, applied in the direction of organic chemistry, can solve the problems of limited application range, low light absorption efficiency, poor emission wavelength matching, etc., and achieve the improvement of light absorption efficiency, high photocuring efficiency, and reduced mobility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
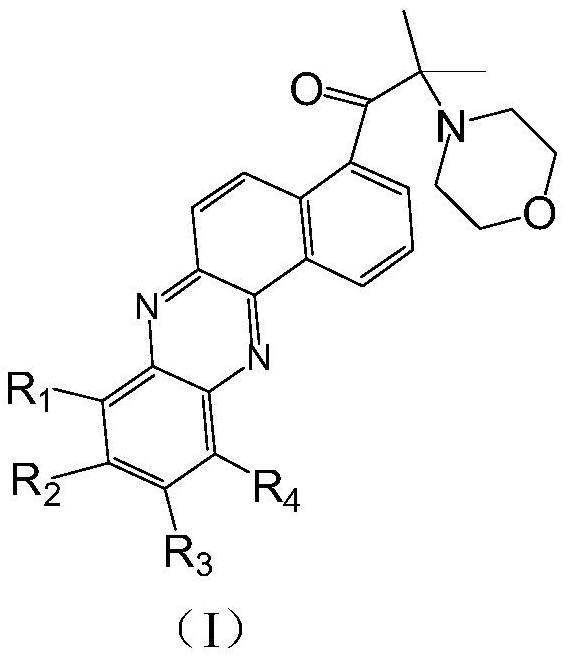
[0094] The molecular formula of the phenazine derivative photoinitiator in the present embodiment is shown in the following formula (II):
[0095]
[0096] The present embodiment provides the preparation method of phenazine derivative photoinitiator as shown above, described method comprises the following steps:
[0097] (1) Disperse 1 mol of 1,2-naphthoquinone and 1.4 mol of aluminum trichloride in 500 mL of dichloroethane, then add 1.2 mol of isobutyryl chloride dropwise, and perform Friedel-Crafts acylation reaction at 13°C for 2 hours, then Mixed with 800mL of 2.5wt% hydrochloric acid for hydrolysis, liquid separation, washing with water, and precipitation to obtain the compound shown in formula (III);
[0098]
[0099] (2) Mix and dissolve the compound represented by formula (III) obtained in step (1) and o-phenylenediamine in acetic acid at a molar ratio of 1:1, and react under stirring at 65°C for 1.5h to remove dissolved to obtain the compound shown in the follo...
Embodiment 2
[0112] The difference between this embodiment and Example 1 is that in the step (2), an equimolar amount of o-phenylenediamine is replaced by 4-methyl o-phenylenediamine, and the molecular formula is as follows;
[0113]
[0114] Other parameters and conditions are exactly the same as in Example 1.
[0115] Include the following two structures in the photoinitiator gained in the present embodiment;
[0116]
[0117] The HPLC content of the two is 1:1;
[0118] The mass spectrometric analysis result of photoinitiator described in the present embodiment is as follows;
[0119] MS: m / z [M+1]+=400.19 (Mw=399.48).
[0120] The H-NMR analysis result of photoinitiator in the present embodiment is as follows;
[0121] 1H-NMR (400MHz, CDCl3): δ8.18~8.10(m,1H),7.95-7.80(m,2H),7.75~7.67(m,3H),7.55(m,1H),7.30~7.20(m ,1H) 3.65(t,4H), 2.40~2.30(m,7H), 1.45(s,6H).
Embodiment 3
[0123] The difference between this example and Example 1 is that in step (2), the equimolar amount of o-phenylenediamine is replaced by 3,4-dimethyl-o-phenylenediamine, and the molecular formula is as follows;
[0124]
[0125] Other parameters and conditions are exactly the same as in Example 1.
[0126] Include the following two structures in the photoinitiator gained in the present embodiment;
[0127] The HPLC content of the two is 1:1;
[0128] The mass spectrometric analysis result of photoinitiator described in the present embodiment is as follows;
[0129] MS: m / z [M+1]+=423.21 (Mw=413.51).
[0130] The H-NMR analysis result of photoinitiator in the present embodiment is as follows; 1H-NMR (400MHz, CDCl ): δ8.08 (d, 1H), 7.85~7.67 (m, 4H), 7.36 (d, 1H) ,7.28(t,1H),3.68(t,4H),2.5~2.40(m,10H),1.45(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com