Plate-type immunofluorescence kit for detecting group reactive antibodies and preparation method of plate-type immunofluorescence kit
An immunofluorescence and antibody detection technology, which is applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of high laboratory requirements, time-consuming, and small volume of Terasaki microplates, and achieve rapid screening and specificity And the effect of good repeatability and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
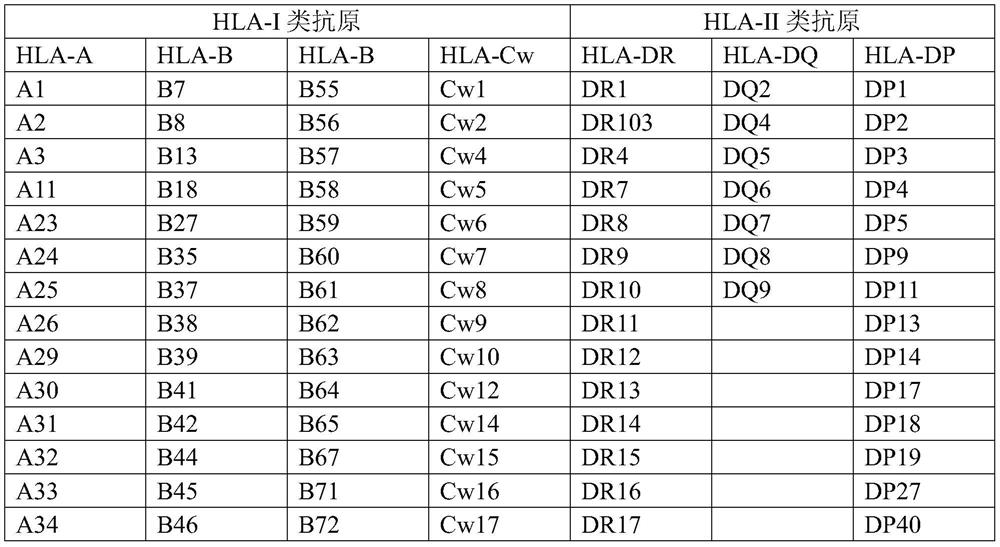
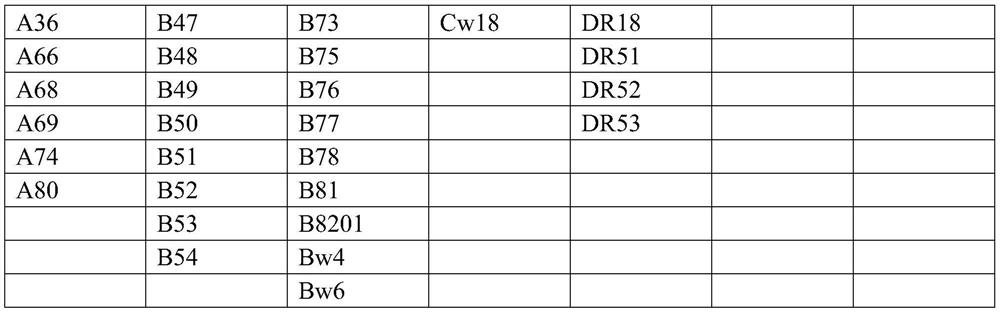
[0031] Example 1 Screening for characteristic dominant sequences of genes encoding HLA-I and HLA-II mixed antigens
[0032] Genes encoding HLA-I and HLA-II class antigens were retrieved from IMGT / HLA.
[0033] The antigenic determinant reactive epitopes of each antigenic protein were predicted by DNAstar-protean, and the gene fragments without reactive epitopes were excluded. Then use NetOGlyc and NetNGlyc to predict the N-glycosylation sites and O-glycosylation sites of the coding genes of each antigen protein, and exclude the reactive epitopes containing N-glycosylation sites and O-glycosylation sites fragments. Due to the high homology between the antigens of HLA-I and II, the fragments with higher homology of antigenic determinant reactive epitopes in the coding genes of the remaining antigenic proteins were selected as HLA-I hybrids. The characteristic dominant sequences of antigens and HLA-II mixed antigens are shown in Table 7:
[0034] Table 7 Characteristic sequenc...
Embodiment 2
[0038] The specific process of protein expression of the characteristic dominant sequence is as follows:
[0039] The designed characteristic dominant sequence was designed and synthesized by General Biosystems Co., Ltd. primers, and the restriction sites of BamH I and Xho I (Takara company) were added to the primer setting, and PCR amplification reaction was carried out to amplify Products were identified using 1.5% agarose gel electrophoresis. The purified PCR product and pcDNA3.1 were digested with BamH I and Xho I, and the separated target gene and plasmid pcDNA3.1 (Biobowell Biotechnology Co., Ltd.) were recovered by gel electrophoresis and treated with T4 DNA ligase (Takara Company) at 16°C. Overnight ligation reaction, take a small amount of linker to transform the competent cell DH5α and culture overnight, and the transformed bacteria are coated with LB solid medium with 100 μg / ml ampicillin (10g / L tryptone, 5g / L yeast extract, 10g / L chloride Sodium, pH=7.4) plates, i...
Embodiment 3
[0042] The protein samples expressed and purified by SEQ ID No.1-17 were diluted to 4 μg / mL and coated on the Elisa plate respectively, and each protein sample was coated with two reaction wells and control wells, 100 μL / well, 4 Coat at ℃ for 18h, wash twice with 200μL / well washing solution, block with 120μL / well blocking solution at 37℃ for 1h, pour off the blocking solution, and pat dry. Add 100 μL of HLA antibody-positive samples of the corresponding proteins in turn to the reaction wells (SEQ ID No.1: A1 positive sample, SEQ ID No.2: A25 positive sample, SEQ ID No.3: A68 positive sample, SEQ ID No.4: B7 Positive sample, SEQ ID No.5: B18 positive sample, SEQ ID No.6: C1 positive sample, SEQ ID No.7: C4 positive sample, SEQ ID No.8: C5 positive sample, SEQ ID No.9: C7 Positive sample, SEQ ID No.10: C17 positive sample, SEQ ID No.11: C18 positive sample, SEQ ID No.12: DR1 positive sample, SEQ ID No.13: DR4 positive sample, SEQ ID No.14: DQ2 Positive sample, SEQ ID No.15: DQ4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com