Synthesis method of thiodicarb
A technology of thiodimethomyl and synthetic methods, applied in the direction of organic chemistry, etc., can solve problems such as product quality impact, insufficient impurity control, easy to block pipelines, etc., to achieve improved selectivity and product quality, simple process operation, and improved selection sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
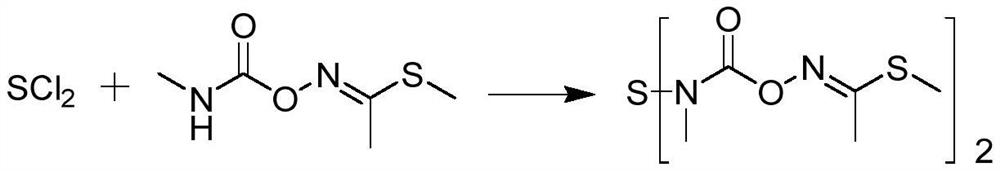
[0032] A kind of synthetic method of thiodicarb of the present invention comprises:
[0033] Add 244.00 g of pyridine and 0.08 g of 18-crown-6 into a 1000 mL four-necked flask equipped with mechanical stirring, a thermometer, and a constant pressure dropping funnel. Seal the reaction system, lower the temperature to -5°C, first dropwise add 1 / 3 of 66.98g sulfur dichloride (calculated as 83% in mass content), after the addition of sulfur dichloride is completed, slowly pass chlorine gas, and then gradually increase the temperature In the process of reaching 15°C, the pyridine solution of methomyl (162.20g methomyl dissolved in 243.00g pyridine) and the remaining sulfur dichloride were added dropwise at the same time, and the dripping was completed within 4 hours, and the chlorine gas flow was stopped after the addition was completed. A total of 0.67g of chlorine gas was passed into the reaction solution, and then kept at 15°C for 4 hours. After the reaction was completed, 285.0...
Embodiment 2
[0036] A kind of synthetic method of thiodicarb of the present invention comprises:
[0037] Add 487.00 g of pyridine and 0.81 g of 18-crown-6 into a 1000 mL four-necked flask equipped with mechanical stirring, a thermometer, and a constant pressure dropping funnel. Seal the reaction system, drop the temperature to 5°C, add dropwise 1 / 2 of 74.43g sulfur dichloride (mass content is calculated according to 83%), after the sulfur dichloride has been added dropwise, start slowly flowing chlorine gas, and then gradually heat up to In the process of 25 DEG C, add dropwise the pyridine solution of methomyl (162.20g methomyl is dissolved in 324.00g pyridine to gain) and remaining sulfur dichloride simultaneously, dropwise finish within 6 hours, stop feeding chlorine gas after dropwise addition, a total of Introduce 2.23g of chlorine gas into the reaction solution, and then keep it warm at 25°C for 6 hours. After the reaction, add 285.00g of methanol to the reaction solution for quench...
Embodiment 3
[0040] A kind of synthetic method of thiodicarb of the present invention comprises:
[0041]Add 373.06 g of pyridine and 0.65 g of 18-crown-6 into a 1000 mL four-neck flask equipped with mechanical stirring, a thermometer, and a constant pressure dropping funnel. Seal the reaction system, drop the temperature to 5°C, add 1 / 2 of 70.70g sulfur dichloride (calculated according to 83% mass content) dropwise, after the sulfur dichloride has been added dropwise, start slowly flowing chlorine gas, and then gradually heat up to In the process of 20 DEG C, dropwise the pyridine solution of methomyl (162.20g methomyl is dissolved in 275.74g pyridine and obtain) and remaining sulfur dichloride simultaneously, dropwise finish within 5 hours, stop logical chlorine gas after dropwise addition, altogether Introduce 1.27g of chlorine gas into the reaction solution, and then keep it warm at 20°C for 5 hours. After the reaction, add 285.00g of methanol to the reaction solution for quenching. Du...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com