Construction method and clinical application of novel non-viral vector TSCM gene therapy
A construction method, PB-CAR-TSCM technology, applied in antiviral agents, antibody medical components, biochemical equipment and methods, etc., can solve problems such as short survival time, weak self-renewal ability, poor therapeutic effect, etc., to reduce Cost of production, avoidance of magnetic bead sorting, effects of improved viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
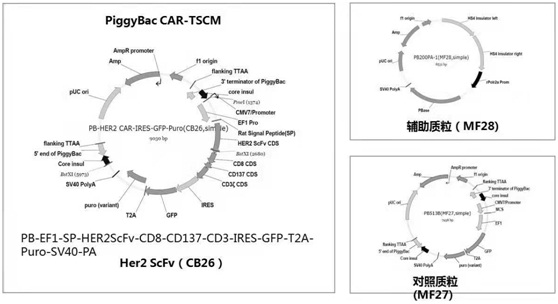
[0041] Construction of the plasmid system:
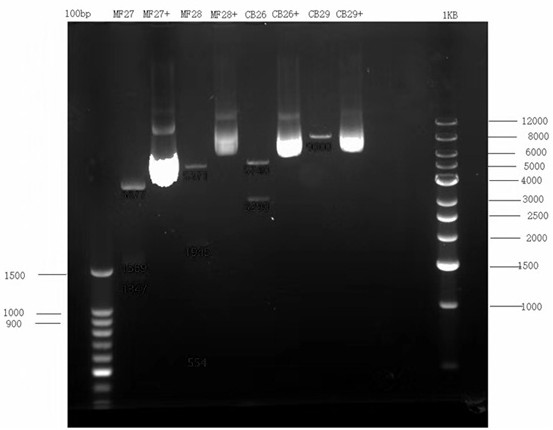
[0042] Construction of PB-EF1-SP-HER2ScFv-CD8-CD137-CD3-IRES-GFP-T2A-Puro-SV40-PA and PB-EF1-SP-CD19ScFv-CD8-CD137-CD3-IRES-GFP-T2A-Puro-SV40 -PA, enzyme digestion identification and sequencing to verify the correctness of the sequence, large plasmid extraction, plasmid digestion electrophoresis detection, all plasmids are correct, see figure 1 and figure 2 .
Embodiment 2
[0044] Tumor cell line establishment and labeling
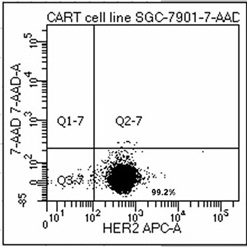
[0045] CD19 positivity of Raji cell line and HER2 positivity of SGC-7901 verified by flow cytometry, HER2 expression was up to 99.2% in SGC-7901 cell line, see Figure 4 .
Embodiment 3
[0047] Preparation of TSCM cells
[0048] 100ml of sodium citrate anticoagulated blood was collected, PBMC and UBMC were separated by lymphocyte separation medium, T cells were purified, and cryopreserved. On day D0, TSCM-II was coated into a culture flask, and placed flat in a 37° C. incubator for more than 2 hours. PBMC and UBMC or T cells were resuspended with TSCM-III according to the cell density of 1×106 / mL, and the TSCM cells were expanded and cultured with TSCM-I medium the next day, after 12-15 days of expansion culture, harvested TSCM cells that meet clinical application standards, and the cell preparations are tested for endotoxin, activity and phenotype. The results of cell identification show that the cell activity is over 95%, and it contains a relatively high proportion of CD3+-BV421 / CD4+-APC-Cy / CD8+APC / CD62L+PE-cy7 / CD45RO-Percp-Cy5.5CD / 45RA+FITC / CD95+PE cells;
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap