Bivalent live vaccine for bovine viral diarrhea and infectious bovine rhinotracheitis and preparation method of bivalent live vaccine
A technology for bovine viral diarrhea and rhinotracheitis, applied in biochemical equipment and methods, viruses, vaccines, etc., can solve the problems of few vaccines, expensive vaccines, and inability to obtain them quickly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be described in detail below in conjunction with the accompanying drawings and examples.
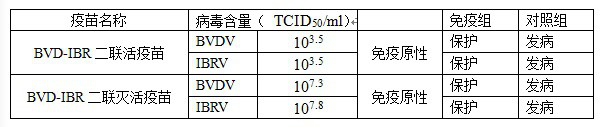
[0031] 1. Vaccine preparation
[0032] 1. Seedling strains
[0033] Bovine viral diarrhea / Mucosal Disease (Bovine viral diarrhea / Mucosal Disease, BVD / MD) NMM strain and bovine infectious rhinotracheitis (Infectious bovine rhinotracheitis, IBR) JSM strain are both preserved by Warwick (Jiangsu) Biopharmaceutical Co., Ltd. .
[0034] Among them, the type I bovine viral diarrhea virus strain was collected from a cattle farm in Inner Mongolia that was suspected to be sick (positive by PCR). One strain of the virus was isolated after the serum was inoculated with MDBK cells. It was detected by RT-PCR, gene sequencing, and neutralization. Test and immunofluorescence test, confirmed that the virus is a BVDVI type virus, named BVDV1 NM01 strain. In order to obtain virus seeds for seedling production, the isolated BVDV1 NM01 strain was continuously passaged on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com