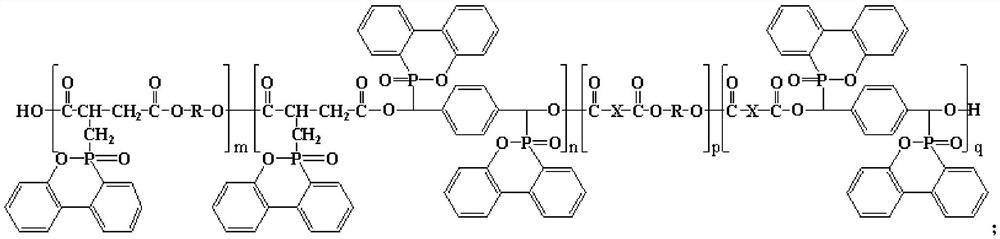
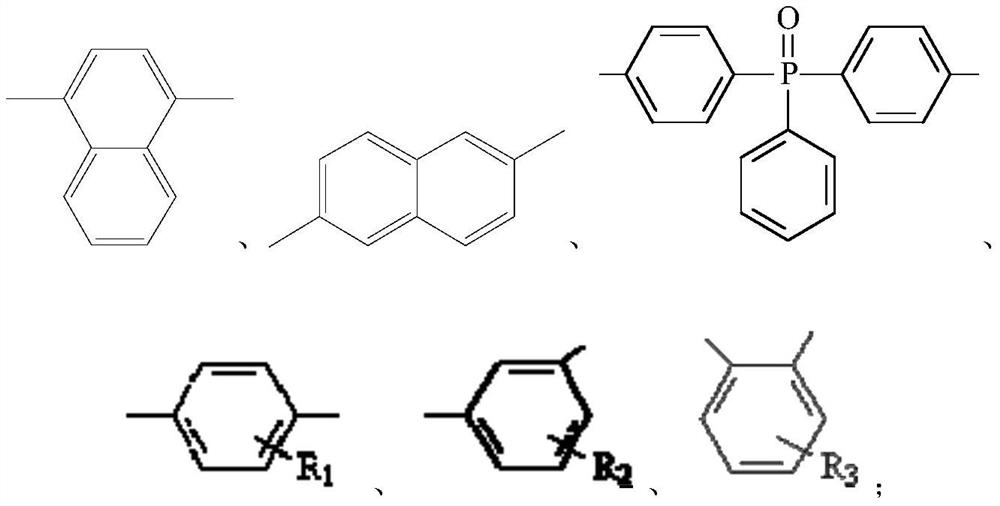
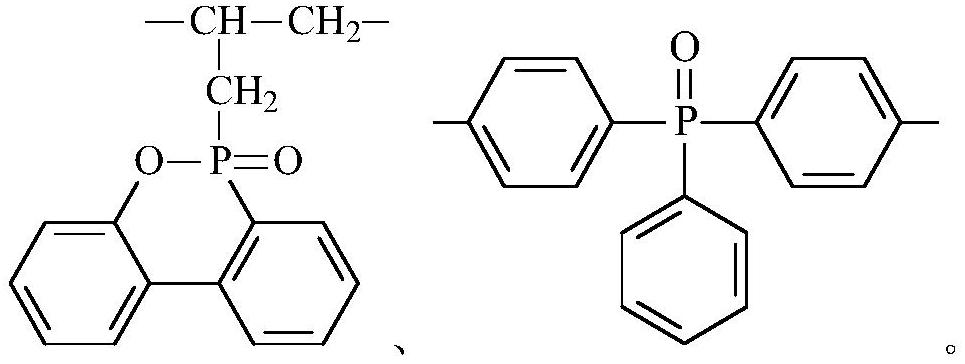
Macromolecular flame retardant, preparation method and application thereof
A technology of flame retardants and macromolecules, used in the preparation and application of flame retardants, can solve problems such as performance loss, complex process, and large reaction steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A preparation method of a macromolecular flame retardant, comprising the steps of:
[0061] (1) Substance A and substance B are added in thionyl chloride respectively, wherein, the amount of substance of thionyl chloride is 3.4 times of the sum of the amount of substance of substance A and substance B, respectively heating Reflux for 0.5 hours, then evaporate to dryness thionyl chloride to obtain the acid chloride of A and the acid chloride of B respectively; wherein, substance A is [(6-oxo-6H-dibenzo[c,e][1,2 ]oxaphosphorin-6-yl)methyl]succinic acid, substance B is HOOC-COOH;
[0062] (2) Mix the acid chloride of A, the acid chloride of B, substance C, substance D, and a catalyst to react, control the reaction temperature to 220°C, and the reaction time is 16 hours, and obtain a macromolecular flame retardant after cooling; Among them, substance C is α,α'-bis(6-oxo-6H-dibenzo[c,e][1,2]oxaphosphorin-6-yl)-1,4-benzenedimethanol , the substance D is D-1, the catalyst is...
Embodiment 2
[0066] A preparation method of a macromolecular flame retardant, comprising the steps of:
[0067] (1) Substance A and substance B are added in thionyl chloride respectively, wherein, the amount of substance of thionyl chloride is 3.1 times of the sum of the amount of substance of substance A and substance B, respectively heating Reflux for 1 hour, then evaporate to dryness thionyl chloride, obtain the acid chloride of A and the acid chloride of B respectively; Wherein, material A is [(6-oxo-6H-dibenzo[c,e][1,2 ]oxaphosphorin-6-yl)methyl]succinic acid, substance B is HOOC(CH 2 ) 4 COOH;
[0068] (2) Mix the acid chloride of A, the acid chloride of B, substance C, substance D and the catalyst to react, control the reaction temperature to 180°C, and the reaction time is 24 hours, and obtain a macromolecular flame retardant after cooling; Among them, substance C is α,α'-bis(6-oxo-6H-dibenzo[c,e][1,2]oxaphosphorin-6-yl)-1,4-benzenedimethanol , the substance D is D-2, and the c...
Embodiment 3
[0071] A preparation method of a macromolecular flame retardant, comprising the steps of:
[0072] (1) Substance A and substance B are added in thionyl chloride respectively, wherein, the amount of substance of thionyl chloride is 9.4 times of the sum of the amount of substance of substance A and substance B, respectively heating Reflux for 5 hours, then evaporate to dryness thionyl chloride, obtain the acid chloride of A and the acid chloride of B respectively; Wherein, material A is [(6-oxo-6H-dibenzo[c,e][1,2 ]oxaphosphorin-6-yl)methyl]succinic acid, substance B is
[0073] (2) react after mixing the acid chloride of A, the acid chloride of B, substance C, substance D and the catalyst, control the reaction temperature to 200°C, and the reaction time is 20 hours, and obtain the macromolecular flame retardant after cooling; Among them, substance C is α,α'-bis(6-oxo-6H-dibenzo[c,e][1,2]oxaphosphorin-6-yl)-1,4-benzenedimethanol , the substance D is D-3, and the catalyst is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com