Micro-reaction system and method for continuously preparing 2-methyl-4-amino-5-cyanopyrimidine by using same
A technology of cyanopyrimidine and dimethylaminomethylene, which is applied in the field of continuous preparation of 2-methyl-4-amino-5-cyanopyrimidine and micro-reaction systems, can solve the problems of long reaction time, complicated operation and high energy consumption. The problem of high consumption is to achieve the effect of shortened reaction time, excellent mass and heat transfer, and small reaction volume.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
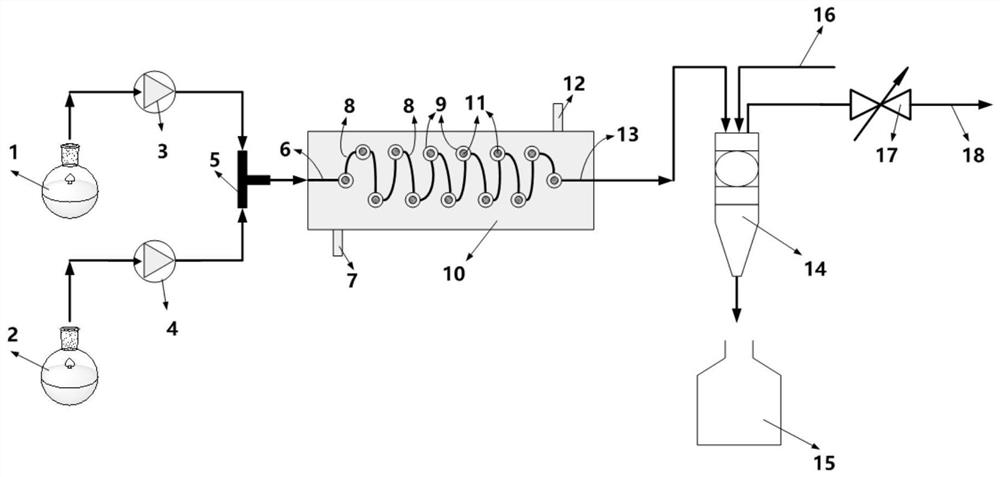
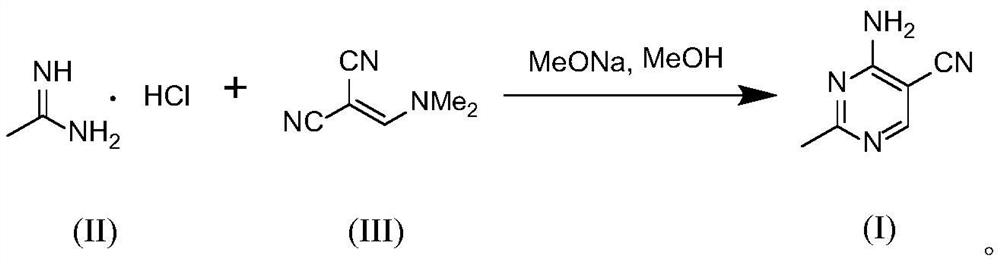
[0076]The methanol solution (200ml) containing sodium methoxide (26.5g, 0.49mol) was cooled to 0°C, and acetamidine hydrochloride (total 46.3g, 0.49mol) was added in batches. After the addition, stir at 0°C for 20min, filter, pour the filtrate into a conical flask, then mix the filtrate with (dimethylaminomethylene) malononitrile (53g, 0.44mol) at a flow rate of 1.12ml / min Methanol solution (350ml) is pumped into the T-type micro-mixer simultaneously with the flow rate of 2.01ml / min (under this condition, the mol ratio of acetamidine hydrochloride and (dimethylaminomethylene) malononitrile is 1:0.92), The temperature in the T-type micro-mixer was controlled to be 55°C, and the two reaction liquids were mixed by the T-type micro-mixer and then directly entered into the Coflore Agitated Cell Reactor (Coflore Agitated Cell Reactor, AMTechnology, UK). The reaction volume inside is 94ml, the vibration frequency is set to 5Hz, the temperature inside the reactor is controlled to 55°C...
Embodiment 2
[0078] This embodiment is the same as Embodiment 1, the only difference is that the temperature inside the T-shaped micro-mixer is controlled to be 35° C. in this embodiment. In this example, the conversion rate of (dimethylaminomethylene) malononitrile was 99%, and the yield of the product 2-methyl-4-amino-5-cyanopyrimidine was 90.4%.
Embodiment 3
[0080] This embodiment is the same as Embodiment 1, the only difference is that the temperature inside the T-shaped micro-mixer is controlled to be 65° C. in this embodiment. In this example, the conversion rate of (dimethylaminomethylene) malononitrile is greater than 99%, and the yield of the product 2-methyl-4-amino-5-cyanopyrimidine is 90.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com