Freeze drying method of azithromycin for injection
A technology of azithromycin and drying method, which is applied in the direction of freeze-drying transportation, drying solid materials, and drying solid materials without heating, etc. It can solve the problems of large difference in moisture content of azithromycin freeze-dried agent, poor uniformity of formed powder, low solubility of azithromycin, etc. , to achieve the effects of promoting conduction and heat conduction, fast forming speed, accelerated sublimation rate and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
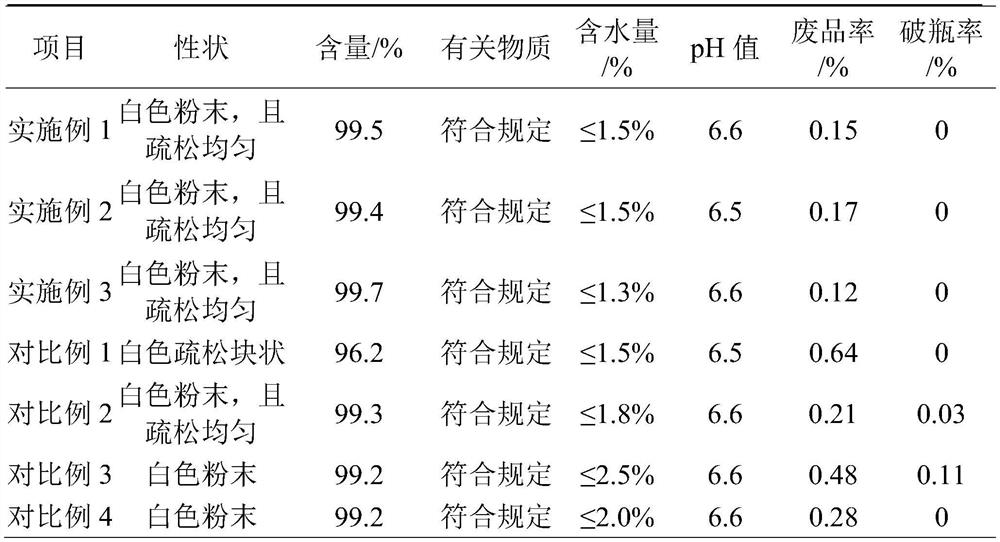
Embodiment 1
[0023] Embodiment 1-a kind of freeze-drying method of azithromycin for injection comprises the steps:
[0024] Step 1: Prepare the solution of azithromycin before lyophilization: mix azithromycin with 25% citric acid solution and 73% citric acid-sodium hydroxide mixed solution in turn, stir until clear, and use the remaining amount of citric acid solution. The citric acid solution is used as a pH regulator to adjust the pH to 6.60, and the rest is supplemented with water for injection to obtain the solution of azithromycin before lyophilization;
[0025] Among them, the prescription volume ratio of azithromycin, anhydrous citric acid, sodium hydroxide and water for injection is: 500:394:190:5548;
[0026] The citric acid-sodium hydroxide mixed solution is to add 700rmp / min of sodium hydroxide to 700rmp / min stirring and dissolving at room temperature 26 ℃ by the water for injection of prescription quantity 20%, drop to 8 ℃, add the anhydrous citric acid of prescription quantity...
Embodiment 2
[0030] Embodiment 2-a kind of freeze-drying method of azithromycin for injection comprises the steps:
[0031] Step 1: Prepare the solution of azithromycin before lyophilization: mix the azithromycin with 24.5% citric acid solution and 73.5% citric acid-sodium hydroxide mixed solution in turn, stir until clear, and use the remaining amount of citric acid solution. The citric acid solution is used as a pH regulator to adjust the pH to 6.60, and the rest is supplemented with water for injection to obtain the solution of azithromycin before lyophilization;
[0032] Among them, the prescription volume ratio of azithromycin, anhydrous citric acid, sodium hydroxide and water for injection is: 540:426:190:5548;
[0033]The citric acid-sodium hydroxide mixed solution is to add 700rmp / min of sodium hydroxide at room temperature 26 ℃ to the water for injection of 20% of the prescription quantity, stir and dissolve, then drop to 16 ℃, add anhydrous citric acid of 73.5% of the prescriptio...
Embodiment 3
[0037] Embodiment 3-a kind of freeze-drying method of azithromycin for injection comprises the steps:
[0038] Step 1: Prepare the solution of azithromycin before freeze-drying: mix the azithromycin with 24.8% citric acid solution and 73.2% citric acid-sodium hydroxide mixed solution successively, stir until clear, and use the remaining amount of citric acid solution. The citric acid solution is used as a pH regulator to adjust the pH to 6.60, and the rest is supplemented with water for injection to obtain the solution of azithromycin before lyophilization;
[0039] Among them, the prescription volume ratio of azithromycin, anhydrous citric acid, sodium hydroxide and water for injection is: 520:410:197.5:5769.5;
[0040] The citric acid-sodium hydroxide mixed solution is to add 700rmp / min of sodium hydroxide to 700rmp / min stirring and dissolving at room temperature 26 ℃ by the water for injection of prescription quantity 20%, drop to 12 ℃, add the anhydrous citric acid of pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com