Method for enzymatically producing dl-tyrosine, broad-spectrum amino acid racemase, and application
A technology of tyrosine and racemase, applied in the field of enzyme catalysis, to achieve the effect of good safety, low raw material price and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for enzymatically producing DL-tyrosine, comprising the following steps:
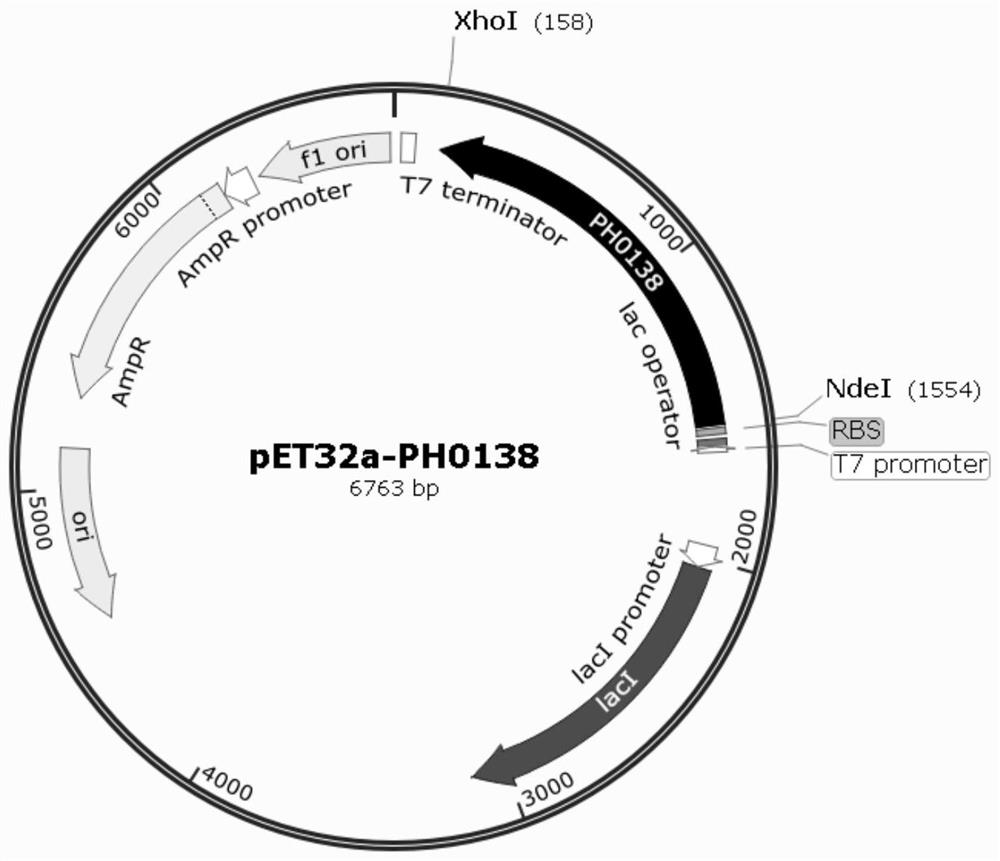
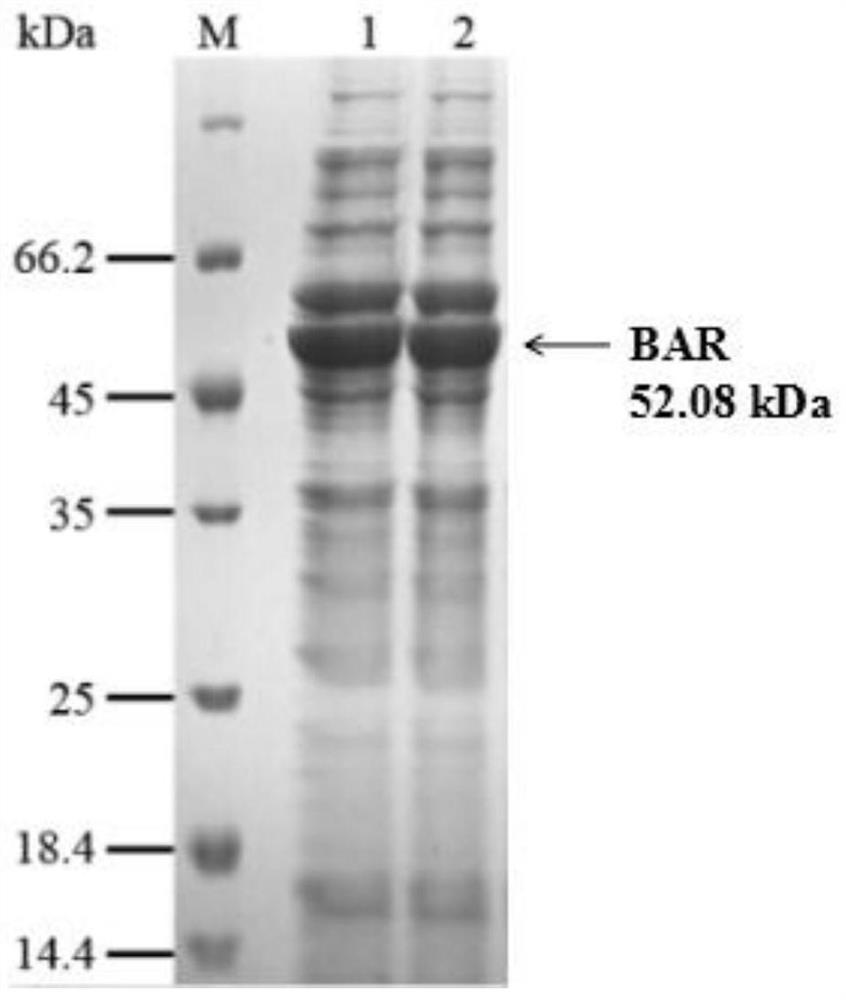
[0042] S1. Construct the recombinant expression vector pET32a-PH0138, transform it into Escherichia coli BL21(DE3) / pG-KJE8, and obtain the recombinant engineered bacterium BBAR;
[0043] S11. According to the GenBank report, the gene sequence encoding broad-spectrum amino acid racemase (BAR) derived from Pyrococcus horikoshii OT3 was codon-optimized to be suitable for exogenous expression in E. coli strains, and the PH0138 gene with optimized sequence was synthesized (SEQ ID NO.1), clone and construct pUC57-PH0138 vector, carrying NdeI and XhoI restriction sites at both ends;
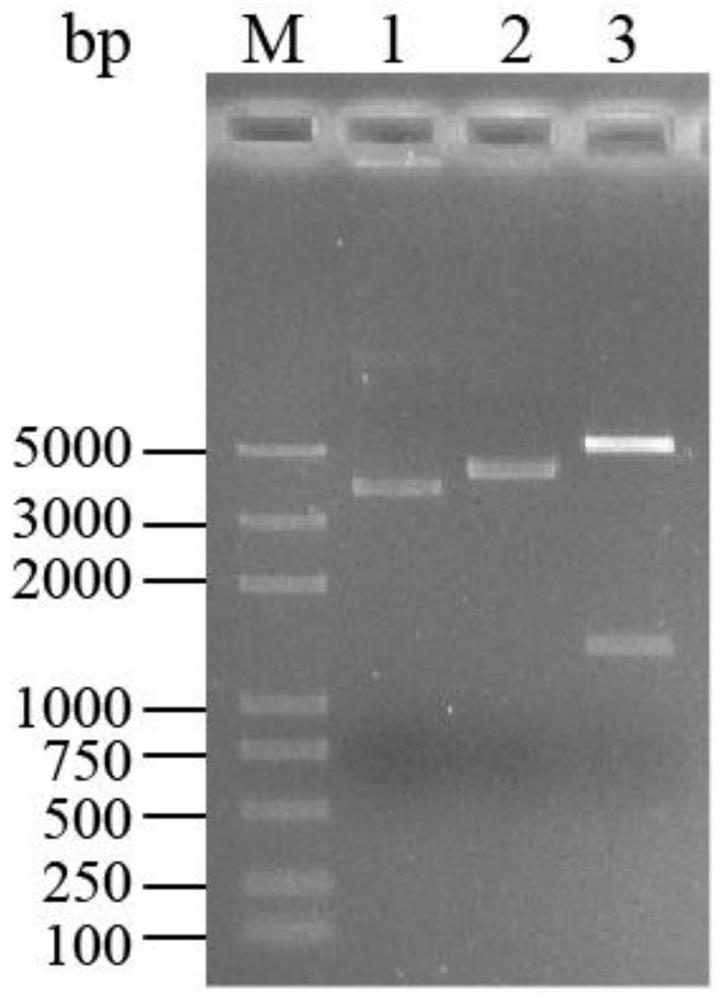
[0044] S12. Digest pUC57-PH0138 vector and pET-32a(+) plasmid with Nde I and Xho I, respectively, use 0.8% agarose gel electrophoresis, cut the gel to recover the target gene fragment and the vector backbone, according to the vector backbone: target gene =1:9 to connect and transform Escherichia coli DH5α compete...
Embodiment 2
[0057] This example is based on Example 1, and the difference from Example 1 is that the biotransformation involved in steps S3-S4 is different, specifically:
[0058] S3. Carry out 600L system conversion in a 1000L conversion tank. The amount of wet bacteria added is 5g / L, weigh 3Kg wet bacteria, resuspend the bacteria with 30L 0.1mol / L potassium phosphate buffer (pH=7.0), and use an ultrasonic pulverizer or a high-pressure homogenizer for cell destruction Obtain BAR crude enzyme liquid afterward, standby;
[0059] S4. Add 30Kg of L-tyrosine, 3Kg of potassium dihydrogen phosphate, and 8.4Kg of dipotassium hydrogen phosphate trihydrate into the 1000L conversion tank, add appropriate amount of water, start stirring, so that L-tyrosine is evenly distributed in the water phase , add BAR crude enzyme solution, then add 15.6g PLP, make up the volume with water to 600L, raise the temperature to 80°C and start stirring transformation, take samples during the transformation process t...
Embodiment 3
[0063] This example is based on Example 1, and the difference from Example 1 is that the biotransformation involved in steps S3-S4 is different, specifically:
[0064] S3. Perform 3000L system conversion in a 5000L conversion tank. The added amount of wet bacteria is 1g / L, weigh 3Kg of wet bacteria, resuspend the bacteria with 30L of 0.1mol / L potassium phosphate buffer (pH=7.0), and use an ultrasonic pulverizer or a high-pressure homogenizer for cell destruction Obtain BAR crude enzyme liquid afterward, standby;
[0065] S4. Add 150Kg of L-tyrosine, 15Kg of potassium dihydrogen phosphate, 42Kg of dipotassium hydrogen phosphate trihydrate into the 5000L conversion tank, add an appropriate amount of water, start stirring, so that L-tyrosine is evenly distributed in the water phase, Add BAR crude enzyme solution, then add 78g of PLP, make up the volume with water to 3000L, raise the temperature to 80°C and start stirring transformation, take samples during the transformation pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com