Electroplating solution for direct cyanide-free copper plating of steel matrix under strong acidic condition and preparation method of electroplating solution
A cyanide-free copper plating and electroplating solution technology, which is applied in the field of direct cyanide-free copper plating electroplating solution and electroplating on steel substrates, can solve the problems of limiting the application range of copper plating products, peeling, etc., and achieve the suppression of side reactions of hydrogen evolution, change of concentration, The effect of improving the bonding force of the coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A direct cyanide-free copper plating electroplating solution for iron and steel substrates under strongly acidic conditions, each component and content are as shown in Table 1;
[0025] The preparation method of this electroplating solution comprises the steps:
[0026] Take 1 / 2 volume of pure water, add copper sulfate to it and stir to dissolve, then add strong acid to it under continuous stirring, then add compound adsorbent and additives in turn and stir evenly, add the rest of pure water to the required volume Electroplating can be carried out after the copper plating solution is obtained.
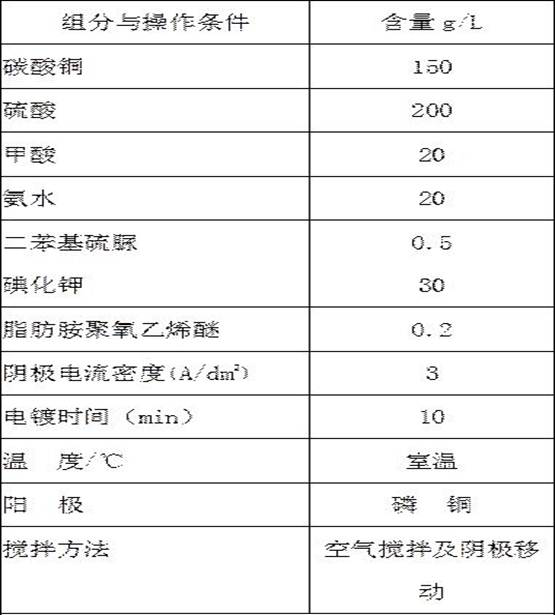
[0027] Table 1 is the electroplating solution of embodiment 1:
[0028] Components and Operating Conditions Contentg / L Copper chloride 100 sulfuric acid 200 formic acid 20 Phenylthiourea 0.3 ammonium chloride 30 ammonia 70 Herotropine 1 Aliphatic amine polyoxyethylene ether 0.2 Electrode current density (A / dm ...
Embodiment 2
[0036] A direct cyanide-free copper plating electroplating solution for iron and steel substrates under strong acidic conditions, each component and content are as shown in Table 2;
[0037] The preparation method of the electroplating solution includes the following steps: take 1 / 2 volume of pure water, add copper sulfate to it and stir to dissolve, add strong acid to it under continuous stirring, and then add compound adsorbent and additives in turn and stir evenly , add the remaining amount of pure water to the required volume to obtain a copper plating solution, and then perform electroplating.
[0038] The copper plating solution of table 2 embodiment 2:
[0039] Components and Operating Conditions Contentg / L copper sulfate 100 sulfuric acid 150 formic acid 30 Cucurbituril 2 ammonia 20 ammonium bromide 30 ascorbic acid 1 polyethylene glycol 0.3 Cathode current density (A / dm 2 )
3 Plating time (min) ...
Embodiment 3
[0047] A direct cyanide-free copper plating electroplating solution for iron and steel substrates under strong acidic conditions, each component and content are as shown in Table 3;
[0048] The preparation method of this electroplating solution comprises the steps:
[0049] Take 1 / 2 volume of pure water, add copper sulfate to it and stir to dissolve, then add strong acid to it under continuous stirring, then add compound adsorbent and additives in turn and stir evenly, add the rest of pure water to the required volume Electroplating can be carried out after the copper plating solution is obtained.
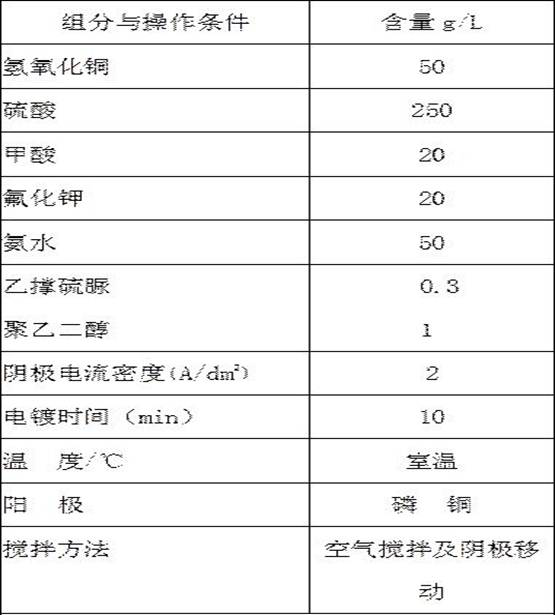
[0050] The copper plating solution of table 3 embodiment 3:
[0051]
[0052] Electroplating method: the steel test piece is degreased, pickled, and activated in sequence, and then placed in the electroplating tank for electroplating, and the current density is set to 3 A / dm 2 , electroplating at room temperature for 10 min to complete the copper plating of steel. It is foun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com