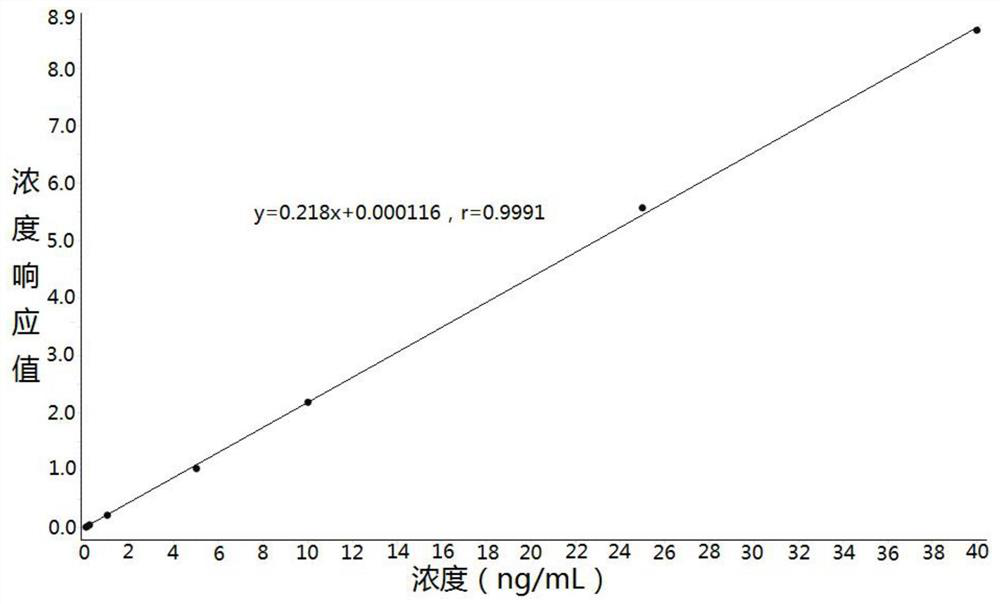
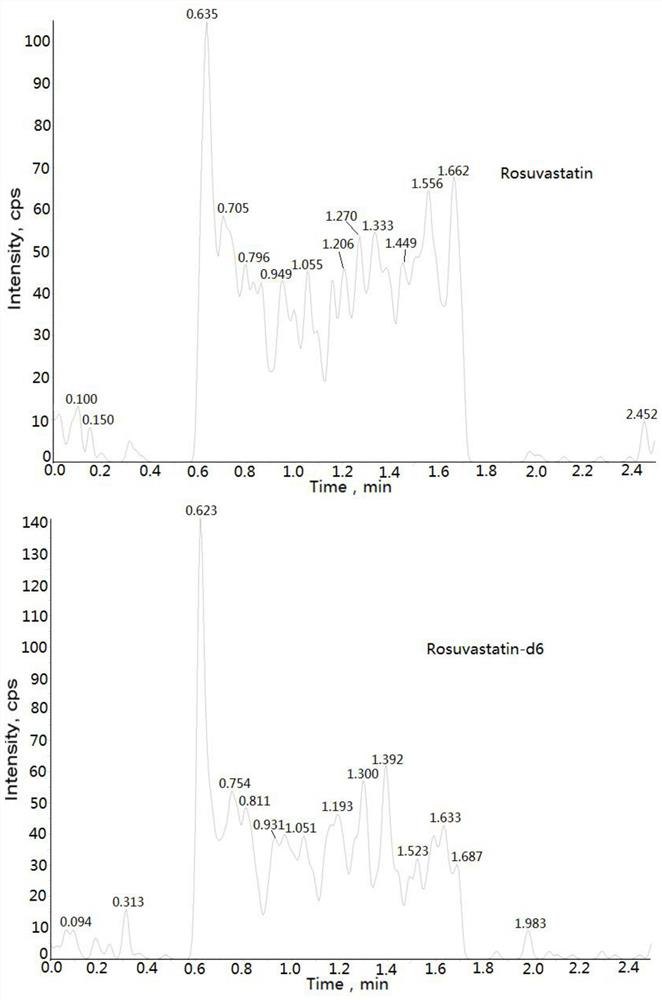
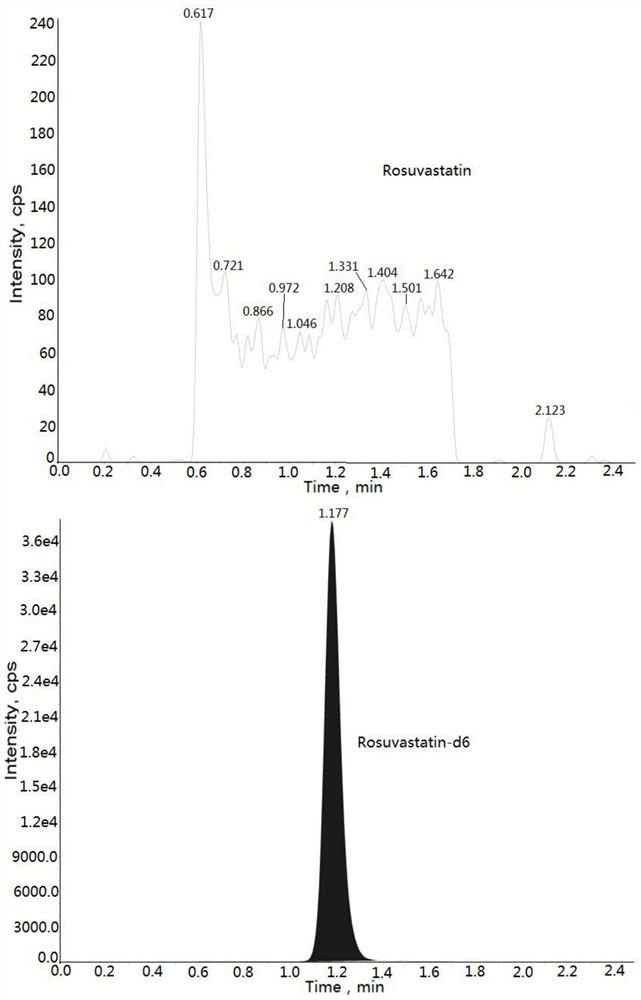
Method for determining concentration of rosuvastatin in blood plasma through liquid chromatography-mass spectrometry
A technology of rosuvastatin and liquid mass spectrometry, which is applied in the field of determination of rosuvastatin concentration in plasma by liquid mass spectrometry, can solve the problems of speed, accuracy, sensitivity, and selectivity to be improved, and achieve high sensitivity and specificity Strong performance and good peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0033] Example: Human K 2 Determination of Rosuvastatin Concentration in EDTA Plasma
[0034] 1. Experimental materials and analytical equipment
[0035] Rosuvastatin (analyte): TLC Pharmaceutical Standards or equivalent or higher grade standard
[0036] Rosuvastatin-d6 (internal standard): TLC Pharmaceutical Standards or the same or higher grade standard
[0037] The reagents used are shown in Table 1 below:
[0038] Table 1 Reagent Details
[0039] Reagent name level manufacturer Methanol (MeOH) HPLC J. T. Baker Formic acid (FA) ACS Adamas Acetonitrile (ACN) HPLC J. T. Baker Potassium dihydrogen phosphate (KH 2 PO 4 )
HPLC Adamas Methyl tert-butyl ether (MTBE) HPLC MREDA
[0040] Note: Reagents of the same grade or higher can also be used
[0041] The analytical equipment used is shown in Table 2 below:
[0042] Table 2 Details of equipment used
[0043]
[0044] The same LC / MS / MS system can also be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com