Artemisinin-chlorambucil ester compound and preparation method thereof
A technology of chlorambucil and chlorambucil, which is applied in the field of artemisinin-chlorambucil synthesis and its preparation, can solve the problems of severe toxic and side effects, achieve low toxic and side effects, reduce Toxic and side effects, strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
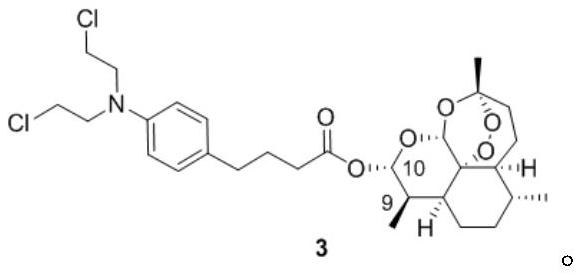
[0020] Embodiment 1 prepares artemisinin-chlorambucil compound
[0021] 1.1 Main instruments and reagents
[0022] Agilent-400M nuclear magnetic resonance instrument (CDCl 3 as solvent, TMS as internal standard, U.S. Agilent Company); Agilent Agilent Accurate-Mass-Q-TOF-MS 6520 mass spectrometer (HRMS-ESI, U.S. Agilent Company); BD FACSCalibur flow cytometer (U.S. Becton-Dickinson company).
[0023] K562 human leukemia cells (Nanjing Kebai Biotechnology Co., Ltd.); K562 / ADR human leukemia cells resistant to doxorubicin (Jiangsu Kaiji Biotechnology Co., Ltd.); dihydroartemisinin, chlorambucil, EDCI, DMAP (Saen Chemical Technology (Shanghai) Co., Ltd.); column chromatography and thin-layer silica gel plate (Qingdao Ocean Chemical Factory); other reagents used were of analytical grade, purchased from Shanghai Titan Technology Co., Ltd.
[0024] 1.2 Synthesis method
[0025] In a 10mL eggplant-shaped bottle, dihydroartemisinin (0.35mmol, 1eq), chlorambucil (1.3eq) and DMAP (3e...
Embodiment 2
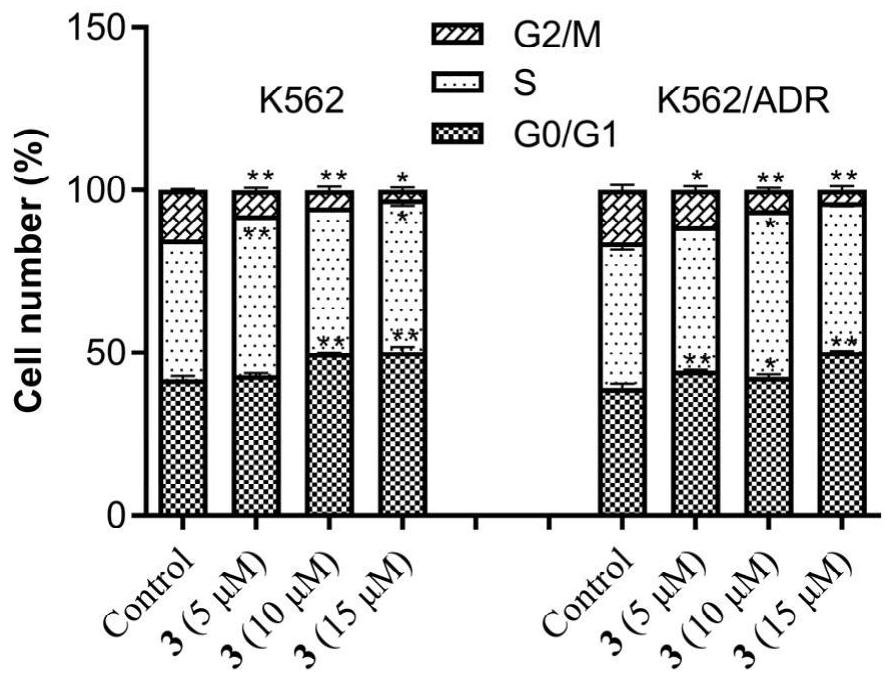
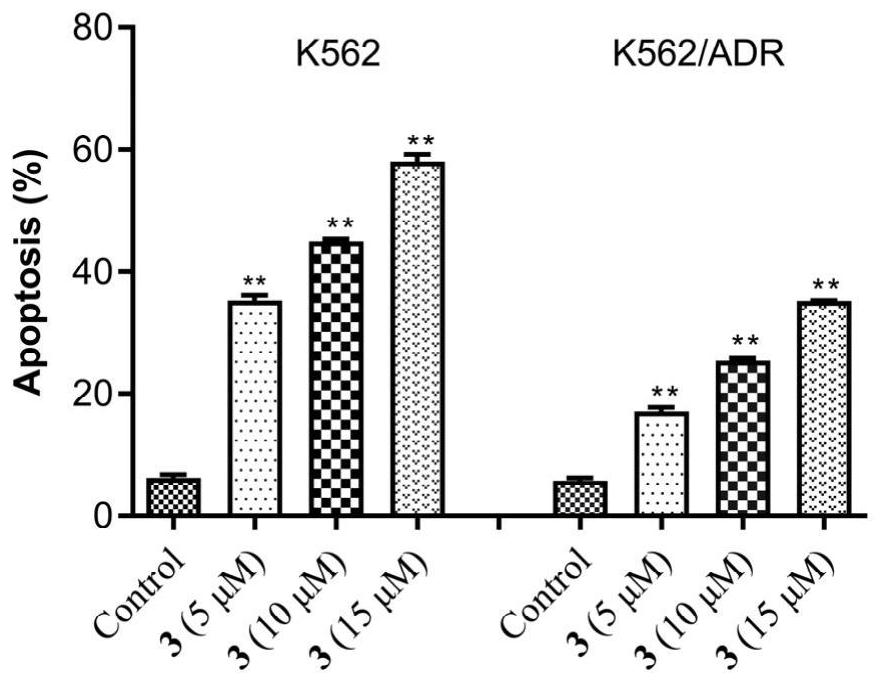
[0028] Embodiment 2 The effect of artemisinin-chlorambucil compound
[0029] 2.1 In vitro anti-tumor activity test
[0030] With doxorubicin as the positive drug, the cytotoxic activity of the target compound on K562 and K562 / ADR was tested by CCK-8 method. Cells were seeded in 96-well plates in 5% CO 2 and incubated at 37°C for 24 hours. Culture media containing different concentrations of compounds were added respectively, and a negative control group was set up at the same time. After incubation for 72 hours, 10 μL of CCK-8 was added to each well, and the culture was continued for 3 hours in the incubator. λ=450nm, read the absorbance value (OD) of each well with a microplate reader, and calculate the inhibition rate. Statistical software SPSS17.0 calculated the half inhibitory concentration (IC 50 )value. The experiment was repeated three times.
[0031] Taking doxorubicin as the positive drug, the in vitro anti-proliferation activity of the new artemisinin-chloramb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com