PARP7 small-molecule inhibitor compound and application thereof
A technology of small molecule inhibitors and compounds, applied in the direction of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of no PARP7 inhibitors, etc., achieve good growth inhibition and good application prospects , Broad spectrum anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Screening and structure of PARP7 inhibitors.
[0073] 1. Construction of pharmacophore and preliminary screening of PARP7 small molecule inhibitors.
[0074] PARP7 inhibitors with high affinity and binding stability to the target protein were obtained through multi-layer virtual screening by computer-aided drug design. Molecular simulation drug design software MOE was used to construct pharmacophore screening and molecular docking. From the 1,767,416 small molecular compounds that have no known reports of PARP7 inhibitory activity derived from the ChemDiv and Specs databases, 11 dominant structures were screened layer by layer for experimental verification, and the PARP7 inhibitor of the present invention was obtained.
[0075] The 3D structure of PARP7 protein was obtained by modeling in the SWISS-MODEL database, and molecular docking was carried out with the active site of PARP7 kinase in the MOE software, and the natural substrate NAD molecule of the prote...
Embodiment 2
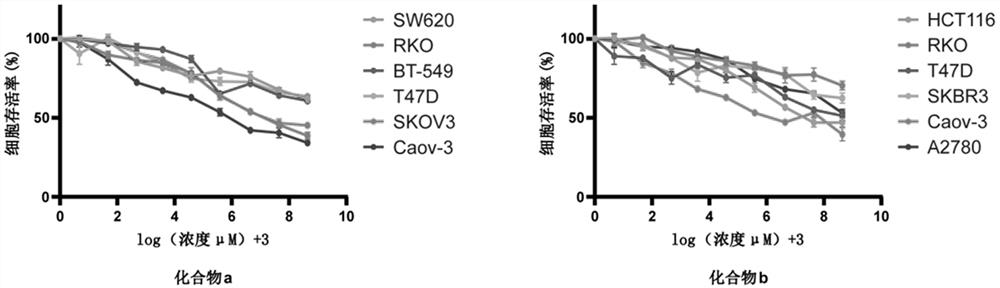
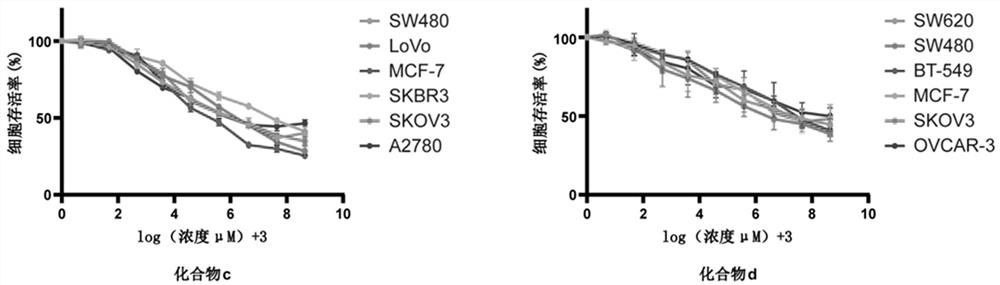
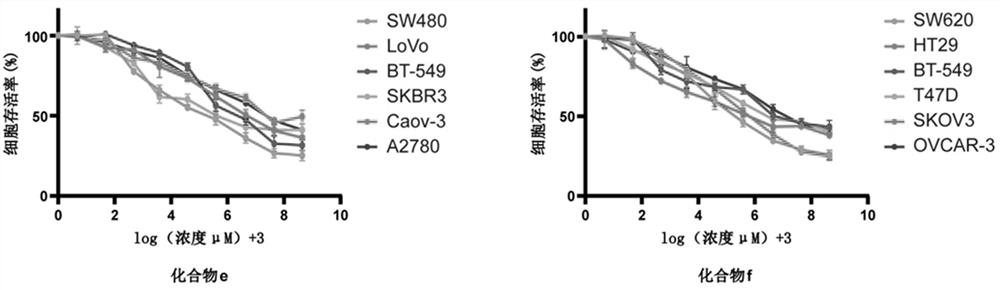
[0083] Example 2 Study on antitumor activity of PARP7 small molecule inhibitor compounds.
[0084] 1. Experimental materials.
[0085] 1.1 Cells and Reagents.
[0086] Human colorectal cancer and intestinal epithelial cell lines: HCT116, SW620, HT29, SW480, RKO, LoVo, NCM460, HIEC; human breast cancer and breast epithelial cell lines: MDA-MB-231, BT-549, SKBR3, MCF-7 , T47D, MCF-10A; human ovarian cancer and ovarian epithelial cell lines: SKOV3, A2780, Caov-3, OVCAR-3, HOSEpiC. DMEM medium, L-15 medium, 1640 medium, McCOY's5A medium, DMEM / F12 medium, MEM medium, trypsin, fetal bovine serum (FBS), dimethylsulfoxide (DMSO), MTT (thiazolium blue).
[0087] 2. Experimental methods and conclusions.
[0088] 2.1 Cell culture.
[0089] Take the cells out of the -80°C deep freezer or liquid nitrogen storage tank, quickly place them in a 37°C water bath to thaw, then transfer the cells to a 2mL centrifuge tube, centrifuge at 1200rmp for 5min, discard the containing Liquid superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com