Method for producing xylanase at high yield from trichoderma reesei and application of xylanase
A technology of Trichoderma reesei and xylanase, applied in the field of bioengineering, can solve problems such as hindering the production of recombinant proteins, achieve the effects of improving saccharification efficiency, enhancing expression ability, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The construction of embodiment 1 recombinant plasmid and Trichoderma reesei recombinant strain
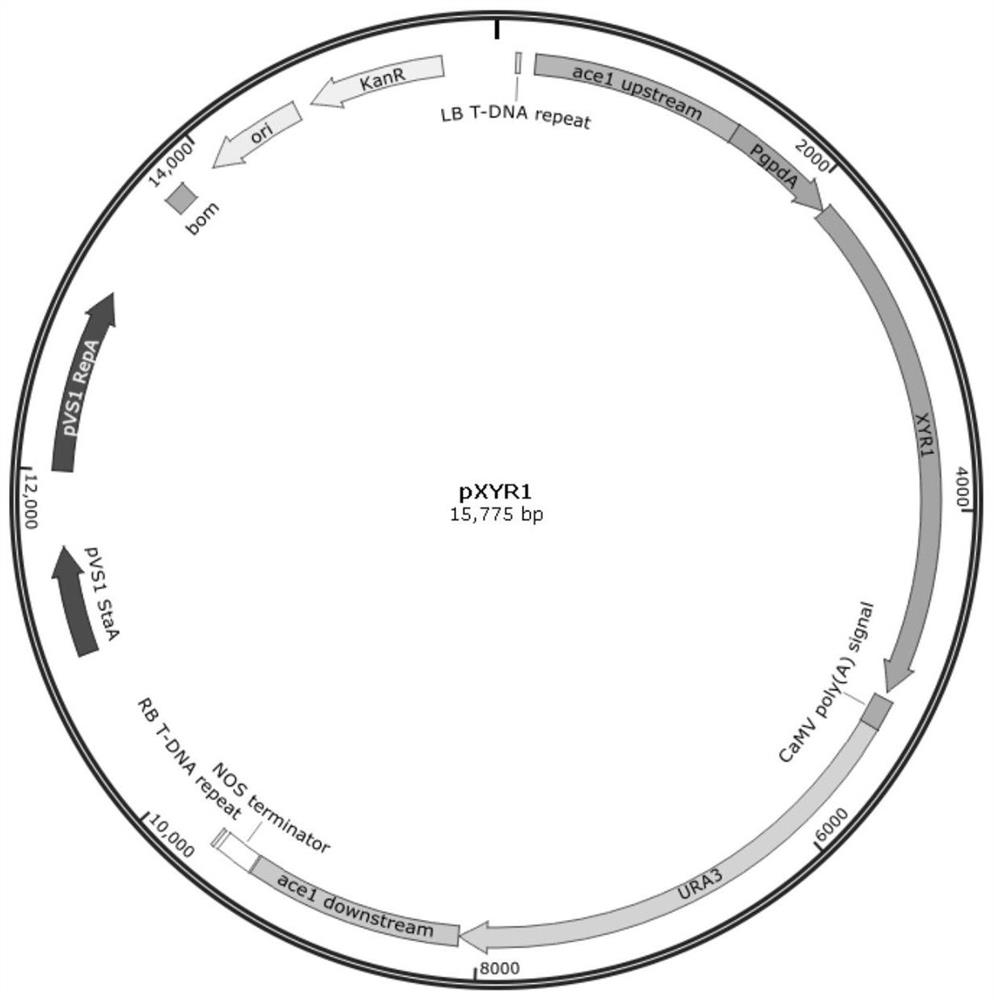
[0068] Using primers P1 and P2, the coding region of XYR1 was amplified using the Trichoderma reesei genome as a template, and connected to the pCAMBIA1301G vector sequence obtained by reverse amplification of primers P3 and P4 through InFusion to construct vector 1 containing the XYR1 expression framework , and use primers P5 and P6 to amplify the region containing the PgpdA promoter and XYR1 coding frame + terminator using vector 1 as a template to obtain the expression frame of XYR1. Next, primers P7 and P8 were used to amplify the URA3 screening marker using vector 2 as a template. Then, using the Trichoderma reesei genome as a template, primers P9 and P10 were used to amplify the upstream of the homology arm of ace1, and primers P11 and P12 were used to amplify the downstream of the homology arm of ace1. The upstream of the homology arm of ace1, the expression frame of...
Embodiment 2
[0072] Efficient secretory expression of embodiment 2 xylanase
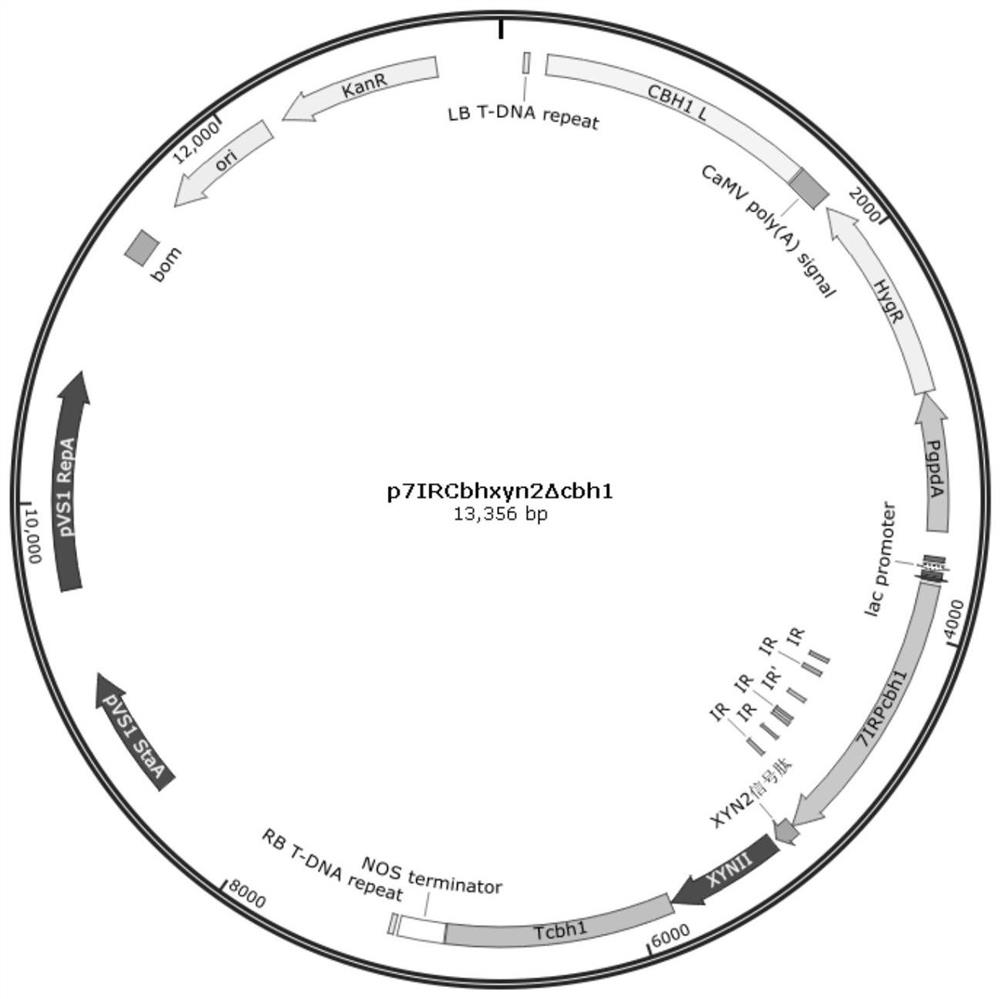
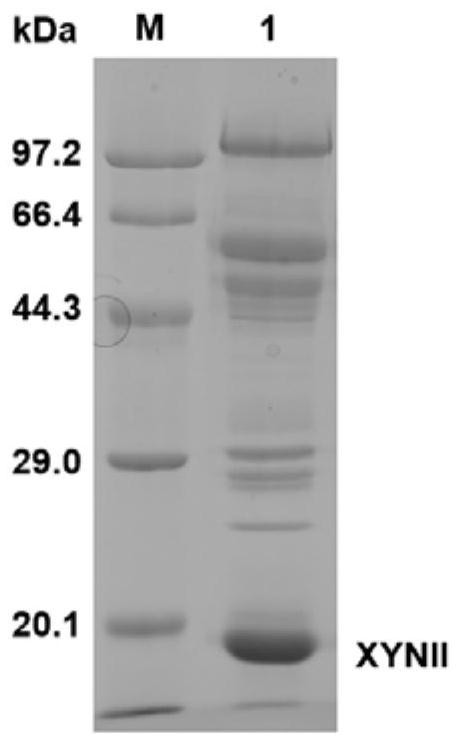
[0073] Inoculate 1 μL of Trichoderma reesei spores to the center of the PDA plate and culture at 30°C for 7 days to form sporulation. Spores were collected with saline and diluted to a concentration of 10 7 / mL. Inoculate 0.5mL 10 7 / mL spores into 20mL SDB medium, cultivated at 28°C 200rpm for 40h, then transferred 5mL of mycelia to the cellulose-induced fermentation medium, cultured at 28°C 200rpm for 4-5 days, collected the fermentation broth by centrifugation, and detected the content of the supernatant of the fermentation broth Xylanase activity and xylosidase activity. The xylanase activity in the fermentation broth of the recombinant strain C30OExyr1 / 7IRxyn2Δcbh1 reached 6410U / mL ( Figure 4 A), the SDS-PAGE of the supernatant of the fermentation broth is as follows image 3 shown. In addition to the improvement of xylanase activity, the improvement of xylosidase activity of C30OExyr1 / 7IRxyn2Δcbh1 ha...
Embodiment 3
[0075] Example 3 Straw Saccharification Experiment Using Xylanase Fermentation Broth Cooperating with Commercial Cellulase
[0076] Inoculate 1 μL of recombinant Trichoderma reesei spores to the center of the PDA plate and culture at 30°C for 7 days to form sporulation. Spores were collected with saline and diluted to a concentration of 10 7 / mL. Inoculate 0.5mL 10 7 / mL spores into 20mL SDB medium, cultured at 28°C 200rpm for 40h, then transferred 5mL mycelia to cellulose-induced fermentation medium, cultured at 28°C 200rpm for 4-5 days, centrifuged to collect the fermentation broth, and obtained crude xylanase Enzyme solution.
[0077] The straw saccharification experiment was carried out with the xylanase crude enzyme solution and the commercial cellulase (Ningxia Xiasheng). The amount of straw added in the saccharification experiment was kept at a solid-to-liquid ratio of 5%, that is, 20 mL of the reaction solution contained 1 g of straw, and the total amount of added ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com