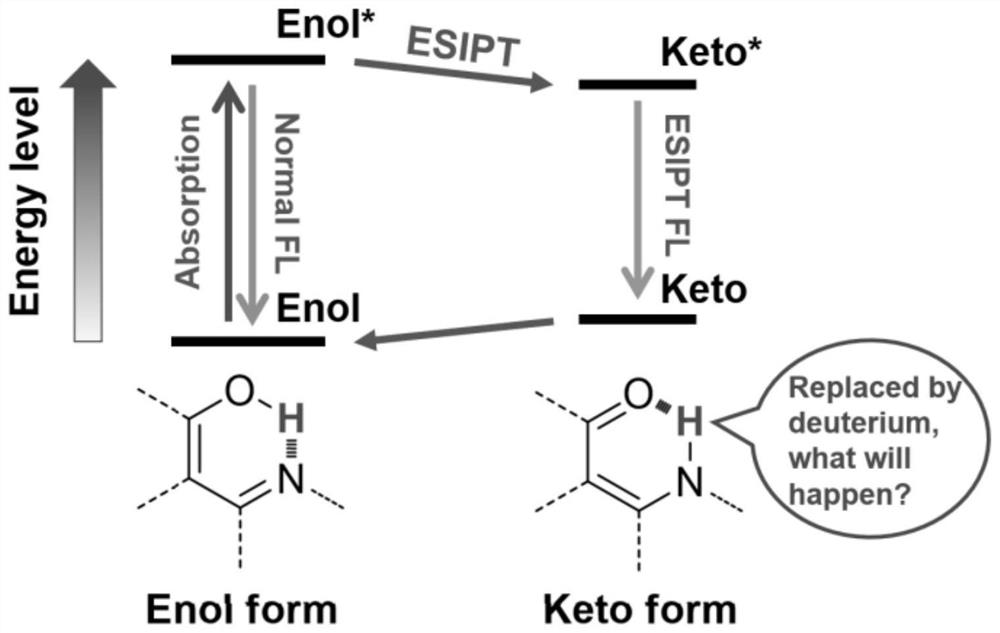
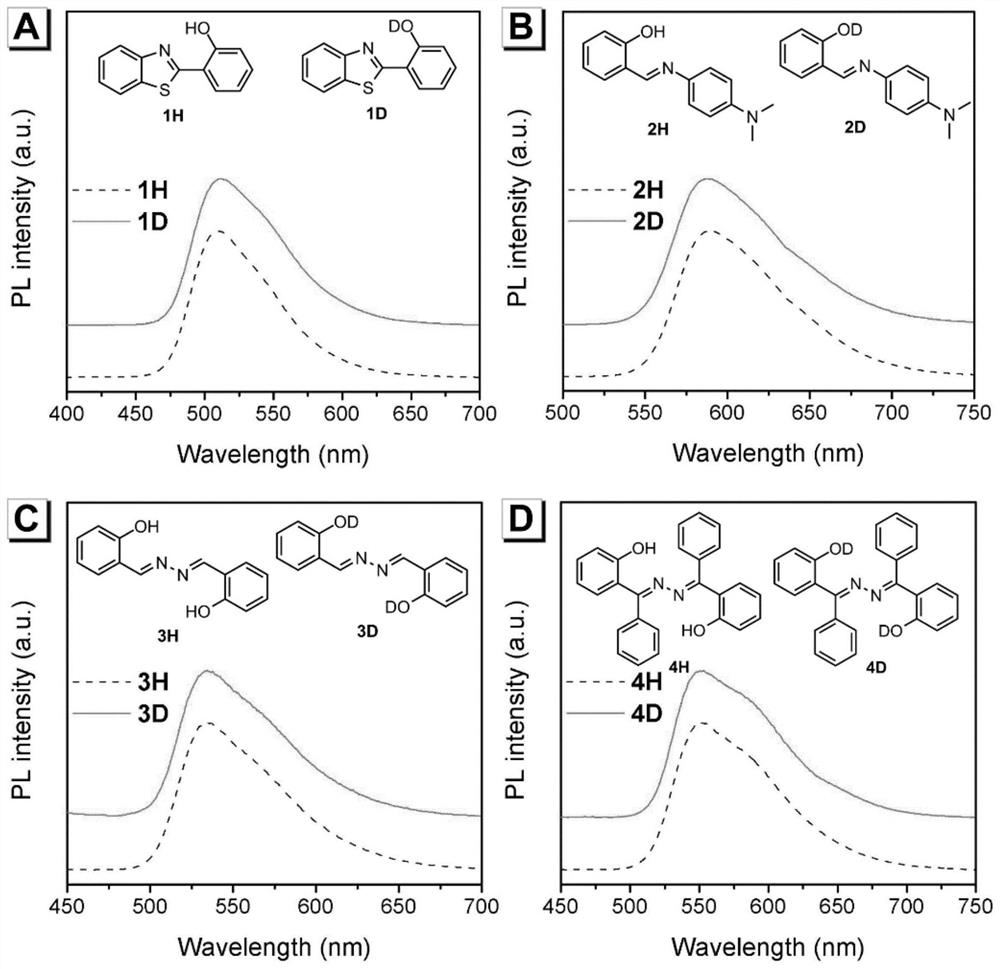
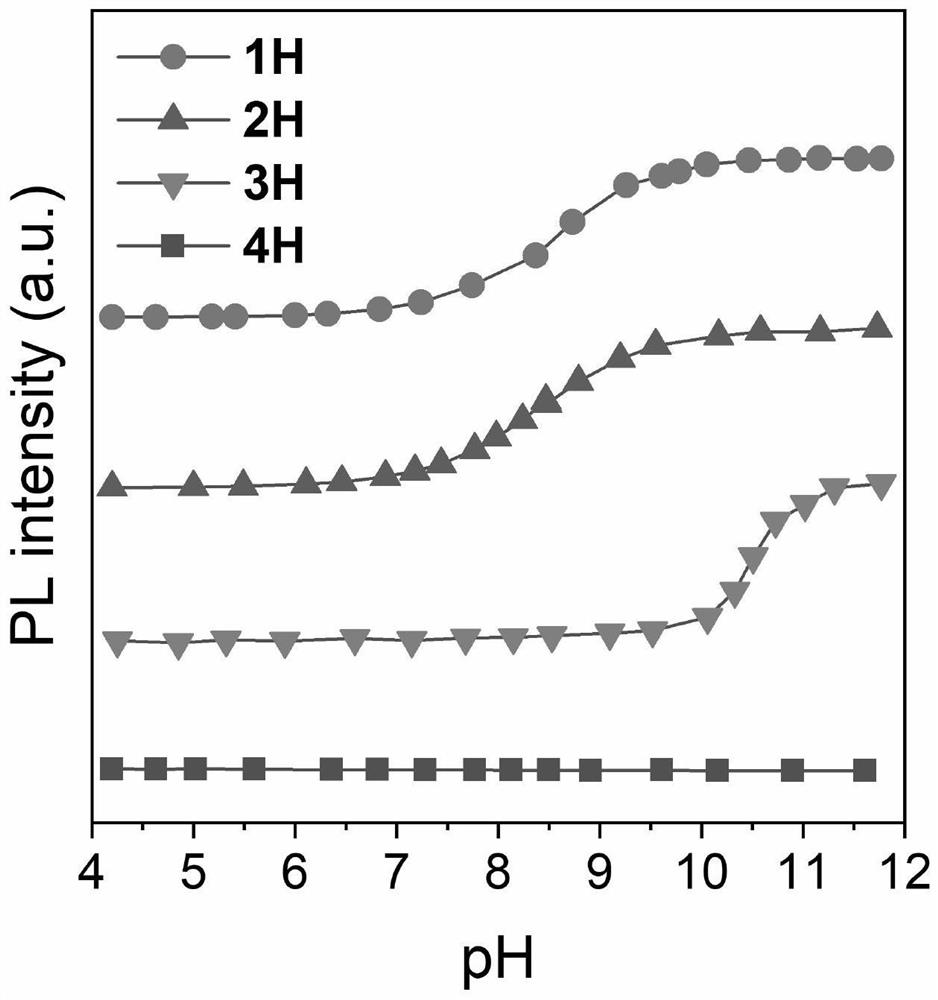
Method for detecting content of heavy water by utilizing aggregation-induced emission molecules
A technology of aggregation-induced luminescence and molecules, which is applied in the field of fluorescent chemical sensors, can solve the problems of excited state dynamics changes, affecting fluorescence lifetime, etc., and achieve the effects of accurate heavy water content, rapid identification, and excellent anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 AIE molecule-1H
[0027] Put 1mmol of aniline derivative (2-aminobenzenethiol) and 1mmol of salicylaldehyde in a 25mL round bottom flask, then add 5mL of absolute ethanol; stir and heat to reflux to 80°C for 1 hour. After the reaction was completed, it was cooled to room temperature, and a precipitate was deposited. Filter and wash the filter cake with 10 mL of ice (0°C) absolute ethanol three times, put the filter cake into a vacuum drying oven, and dry the filter cake under reduced pressure at 60°C to obtain 0.8 mmol of 1H.
[0028] Add the obtained product 1H into a round bottom flask containing 10 mL of tetrahydrofuran, add 5 drops of heavy water, heat and stir until solids are precipitated, and then repeat the replacement process twice. After three replacements, filter with suction, wash the filter cake three times with 15 mL ice-free ethanol, and dry under reduced pressure to obtain 1D.
[0029] The structural formulas and reactio...
Embodiment 2
[0031] Embodiment 2 Preparation of AIE molecule-2H
[0032] Put 1 mmol of aniline derivative (N-N-dimethyl-p-phenylenediamine) and 1 mmol of salicylaldehyde in a 25 mL round bottom flask, and then add 5 mL of absolute ethanol. Stir and heat to reflux to 80°C, and the reaction time is 1 hour. After the reaction was completed, it was cooled to room temperature, and a precipitate was deposited. Filter and wash the filter cake three times with 10 mL of ice (0° C.) absolute ethanol, put the filter cake in a vacuum oven, and dry under reduced pressure at 60° C. to obtain 0.8 mmol 2H of the product.
[0033] Add the obtained product 2H into a round bottom flask containing 10 mL of tetrahydrofuran, add 5 drops of heavy water, heat and stir until solids are precipitated, and then repeat the replacement process twice. After three replacements, filter with suction, wash the filter cake three times with 15 mL of ice-free ethanol, and dry under reduced pressure to obtain 2D.
[0034] Th...
Embodiment 3
[0036] Embodiment 3 Preparation of AIE molecule-3H
[0037] Put 1mmol of hydrazine hydrate and 2mmol of salicylaldehyde in a 25mL round bottom flask, and then add 5mL of absolute ethanol. Stir and heat to 80°C for a reaction time of 30 minutes. After the reaction was completed, it was cooled to room temperature, and a precipitate was deposited. Filter, and wash the filter cake three times with 10 mL of cold (0° C.) absolute ethanol, put the filter cake in a vacuum oven, and dry under reduced pressure at 60° C. to obtain 0.8 mmol 3H of the product.
[0038] Add the obtained product 3H into a round-bottomed flask containing 10 mL of tetrahydrofuran solution, add 5 drops of heavy water, heat and stir until solids are precipitated, and then repeat the replacement process twice. After three replacements, filter with suction, wash the filter cake three times with 15 mL ice-free ethanol, and dry under reduced pressure to obtain 3D.
[0039] The structural formulas and reaction pri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com