CpG medicine and anthracene ring medicine compound, preparation method and application
A drug compound and anthracycline technology, applied in the field of biomedicine, can solve the problems of reducing immune activation of CpG drugs, congestive heart failure, unfavorable immunotherapy, etc., to prevent tumor immune escape, reduce toxic side effects, improve Immunogenic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
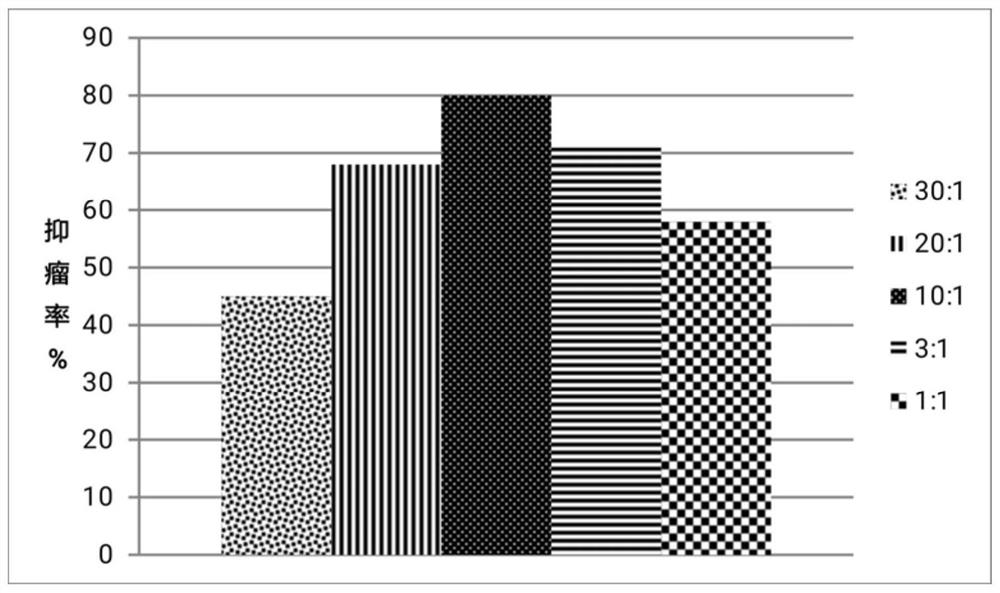
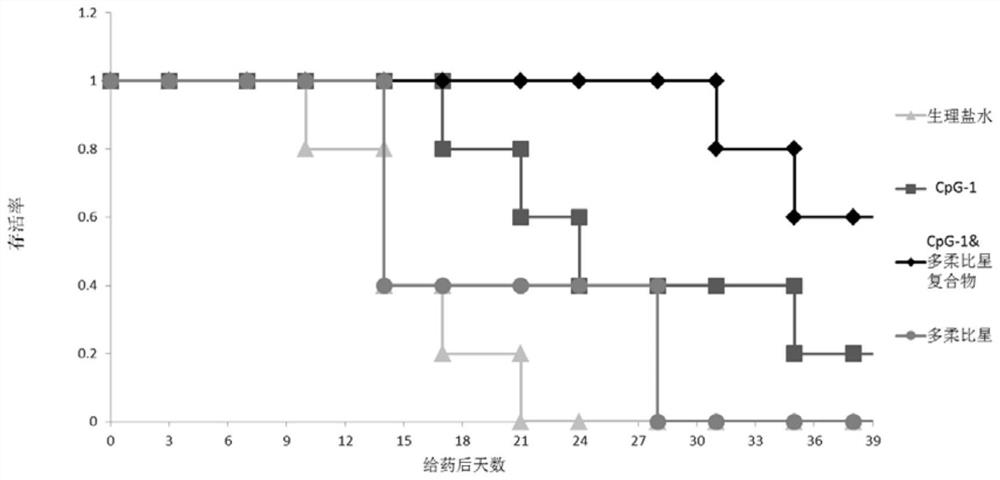
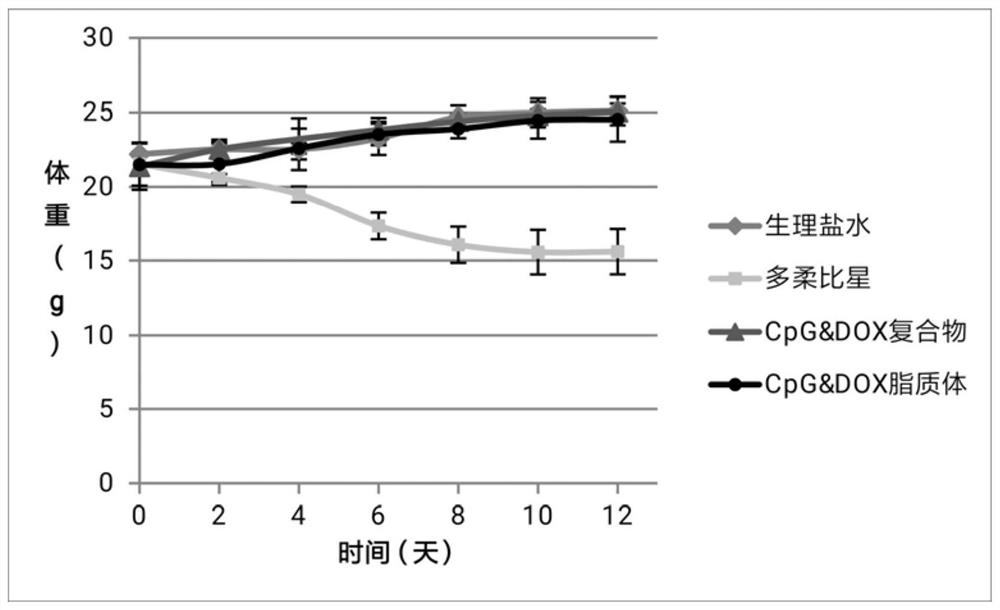
Image
Examples
Embodiment 1
[0038] Embodiment 1: take by weighing 5mg pirarubicin (Dao Zhongdao (Heze) Pharmaceutical Co., Ltd.), be mixed with the pirarubicin stock solution of 2mg / mL with distilled water; Take by weighing 5mg CpG-1 (sequence: 5'-tcgaacgttcgaacgttcgaacgttcgaat -3' (full thiophosphorylation modification of phosphodiester bonds), manufacturer: Guangzhou Ruibo Biotechnology Co., Ltd.) was prepared into 1 mg / mL CpG-1 stock solution with HEPES buffer. Mix 2 mL of CpG-1 stock solution with 0.2 mL of pirarubicin stock solution, and stir at room temperature for 5 min to obtain the complex of CpG-1 and pirarubicin.
Embodiment 2
[0039] Embodiment 2: Weigh 5mg epirubicin (Dao Zhongdao (Heze) Pharmaceutical Co., Ltd.), and prepare 2mg / mL epirubicin stock solution with distilled water; Weigh 5mg CpG-2 (sequence: 5'-gGGGTCGTCGACGAggggG -3' (lowercase letters represent phosphodiester bond modified by phosphorothioation), manufacturer: Guangzhou Ruibo Biotechnology Co., Ltd.) was prepared into 1 mg / mL CpG-2 stock solution with HEPES buffer. Mix 2 mL of CpG-2 stock solution with 0.1 mL of epirubicin stock solution, and stir at room temperature for 2 h to obtain the complex of CpG-2 and epirubicin.
Embodiment 3
[0040] Embodiment 3: Weigh 5mg doxorubicin (hydrochloride form, Daozhongdao (Heze) Pharmaceutical Co., Ltd.), and prepare the doxorubicin stock solution of 2mg / mL with distilled water; Weigh 5mg CpG-2 (sequence : 5'-gGGGTCGTCGACGAggggG-3' (lowercase letters represent phosphodiester bonds modified by phosphorothioation), manufacturer: Guangzhou Ruibo Biotechnology Co., Ltd.) Prepare 1mg / mL CpG-2 stock solution with HEPES buffer . Mix 2 mL of CpG-2 stock solution with 0.33 mL of doxorubicin stock solution, and stir at room temperature for 1.5 h to obtain the CpG-2 and doxorubicin complex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com