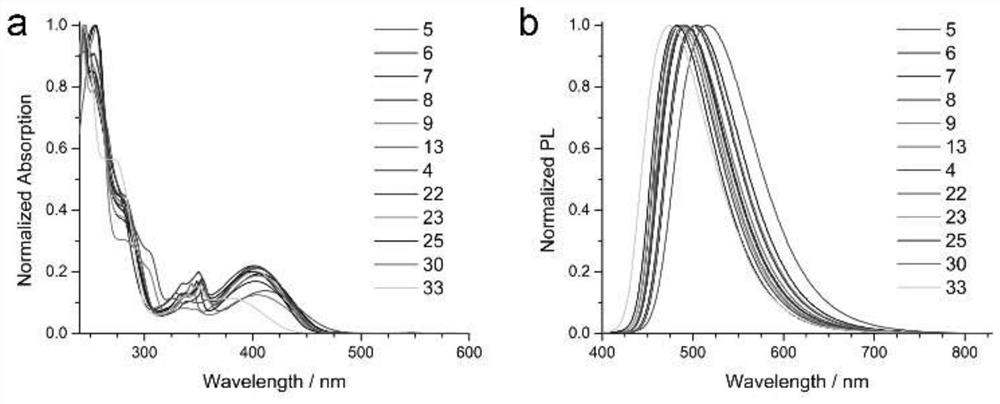
A kind of π-conjugated polycyclic aminoisoquinoline compound and its synthesis method and application
A polycyclic aminoisoquinoline and compound technology, which is applied in the fields of organic chemistry and materials science to achieve the effects of long photoluminescence lifetime, strong fluorescence quantum yield and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Synthesis of π-conjugated polycyclic aminoisoquinolines
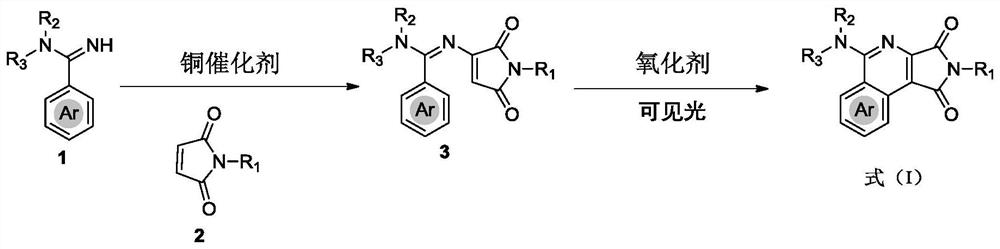
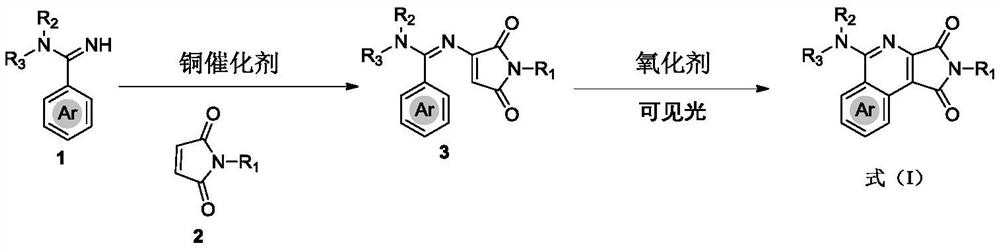
[0047] (1) Synthesis of compound 3 by oxidative amination reaction: weighed aryl amidine 1 (0.25 mmol, 1.0 equiv), maleimide 2 (0.50 mmol, 2.0 equiv), Cu(OAc) 2 (9.0 mg, 20.0 mol%) and 2,2'-bipyridine (11.7 mg, 30.0 mol) were placed in a dry reaction tube, and after vacuum-oxygen replacement (three times) through a double row tube, anhydrous was added. DCE (1.0 mL) was stirred at 120 °C for 12 h under an atmosphere of oxygen (1 atm). The solvent was evaporated in vacuo at room temperature and the remaining residue was purified by silica gel column chromatography (n-hexane / EtOAc as eluent) to afford starting material 3.
[0048] (2) Synthesis of compound 4: Place the weighed raw material 3 (0.15 mmol, 1.0 equiv.), TEMPO (0.03 mmol, 20 mol%) in a dry reaction tube, and carry out vacuum-oxygen replacement through double-row tubes (three times) ), isopropanol (3.0 mL) was added, and the mixture was stirre...
Embodiment 2
[0265] Embodiment 2 explores the influence of solvent selection on the synthesis of intermediate compounds in the process of oxidative amination
[0266] With reference to the preparation process of Example 1, when each reaction condition selects the conditions shown in Table 1, the results of the corresponding synthetic intermediate compound 3-a are shown in Table 1:
[0267] Table 1 Results of the synthesis of intermediate compound 3-a in different oxidative amination reaction solvent systems
[0268]
Embodiment 3
[0269] Example 3 Exploring the influence of catalyst selection on the synthesis of intermediate compounds in the process of oxidative amination
[0270] With reference to the preparation process of Example 1, when each reaction condition selects the conditions shown in Table 2, the results of the corresponding synthetic intermediate compound 3-a are shown in Table 2:
[0271] Table 2 Results of the synthesis of intermediate compound 3-a by different oxidative amination catalyst systems
[0272]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com