Preparation method of high-purity lincomycin hydrochloride
A lincomycin hydrochloride, high-purity technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as difficult removal, achieve drug safety, simplify process flow, and achieve good economic benefits. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
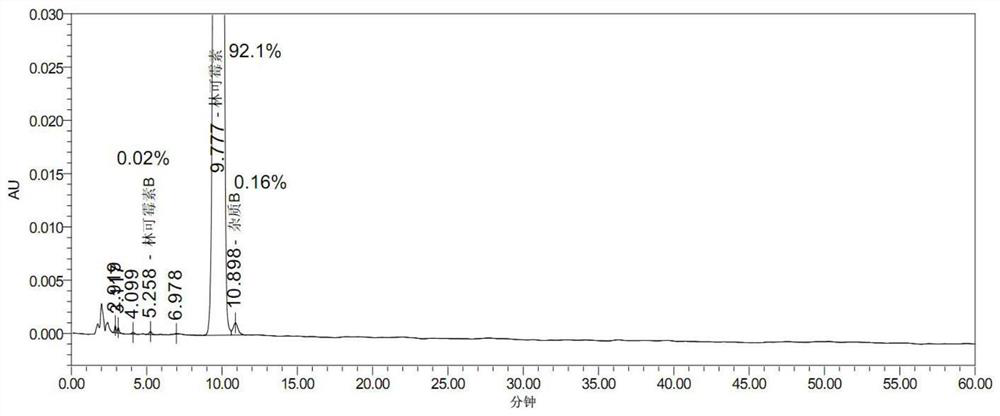
Embodiment 1
[0059] 1) Weigh 16.5kg of potassium dihydrogen phosphate and add 330L of purified water, weigh 390.97kg of lincomycin hydrochloride dry powder, heat up to 72.7°C, keep warm and melt until clarification, slowly add 100kg of sodium chloride, stir and hold at 69.3°C for 35min, then cool down Crystallization, when the temperature drops to 42.7°C, centrifuge and filter at 1500r / min to obtain intermediate wet product 1;
[0060] 2) Put the filtered intermediate wet product 1 back into the dissolution tank, and add 47.62kg / m according to purified water (L): dry powder (wet product is decomposed after moisture detection, kg) = 1:1.128 3 Potassium dihydrogen phosphate, saturated sodium chloride solution, repeat 1) operation step 2 times, obtain 380.9kg lincomycin hydrochloride intermediate wet product 2 altogether.
[0061] 3) Dissolve 380.9kg of lincomycin hydrochloride intermediate wet product 2 in purified water to 64116u / ml, and adjust the pH to 10.27 with 12% NaOH solution;
[00...
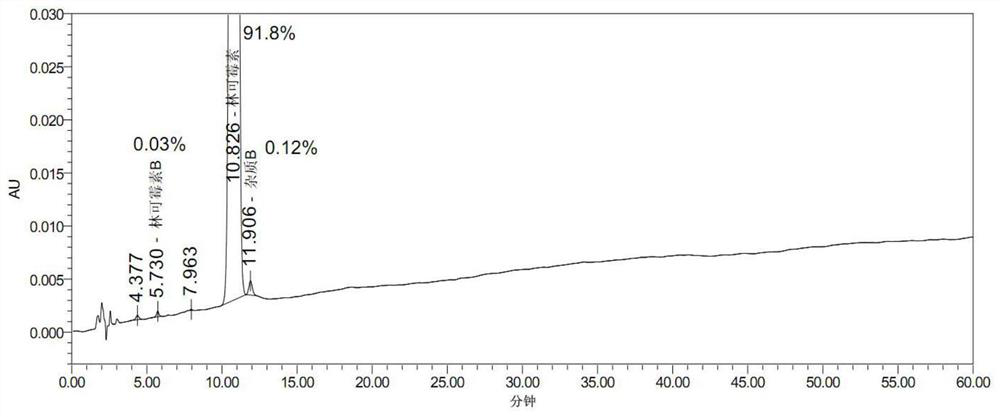
Embodiment 2
[0067] 1) Weigh 16.5kg of potassium dihydrogen phosphate and add 330L of purified water, weigh 386.86kg of lincomycin hydrochloride dry powder, heat up to 63.6°C, keep warm and melt until clarified, slowly add 100kg of sodium chloride, stir and hold at 68.3°C for 30min, then cool down Crystallization, when the temperature dropped to 41.2°C, centrifuged at 1500r / min to obtain intermediate wet product 1;
[0068] 2) Put the filtered intermediate wet product 1 back into the dissolution tank according to purified water (L): dry powder (wet product detection moisture is converted to dry, kg) = 1:1.116, add 47.62kg / m 3 Potassium dihydrogen phosphate, saturated sodium chloride solution repeat 1) operation step 3 times, obtain 350.6kg lincomycin hydrochloride intermediate wet product 2 altogether.
[0069] 3) Dissolve 350.6kg of lincomycin hydrochloride intermediate wet product 2 with purified water to 72371u / ml, and adjust the pH to 10.56 with 15% NaOH solution;
[0070] 4) Flow the...
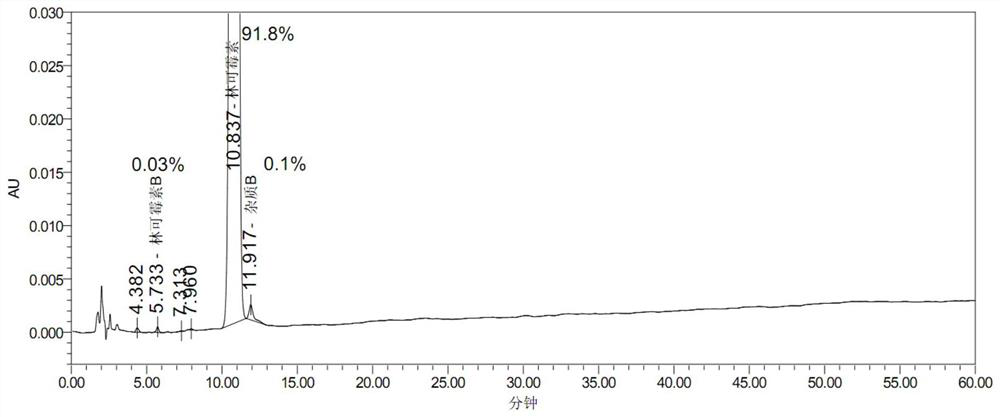
Embodiment 3
[0075] 1) Weigh 16.5kg of potassium dihydrogen phosphate and add 330L of purified water, weigh 397.32kg of lincomycin hydrochloride dry powder, heat up to 78.6°C, keep warm and melt until clarified, slowly add 100kg of sodium chloride, stir and hold at 71.1°C for 30min, then cool down Crystallization, when the temperature drops to 43.5°C, centrifuge and filter at 1500r / min to obtain intermediate wet product 1;
[0076] 2) Put the filtered intermediate wet product 1 back into the dissolving tank according to purified water (L): purified water (L): dry powder (wet product detection moisture converted to dry, kg) = 1:1.146, add 47.62kg / m 3 Potassium dihydrogen phosphate, saturated sodium chloride solution repeat 1) operation step 2 times, obtain 369.8kg lincomycin hydrochloride intermediate wet product 2 altogether.
[0077] 3) Dissolve 369.8kg of lincomycin hydrochloride intermediate wet product 2 in purified water to 59513u / ml, and adjust the pH to 9.78 with 17% NaOH solution; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com