Copper-based catalyst for preparing ethyl alcoholby electrocatalytic reduction of carbon dioxide, and preparation method and application thereof
A copper-based catalyst, carbon dioxide technology, applied in electrodes, electrolysis components, electrolysis process, etc., can solve the problems of ethanol selectivity and current density that have not been reported in research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
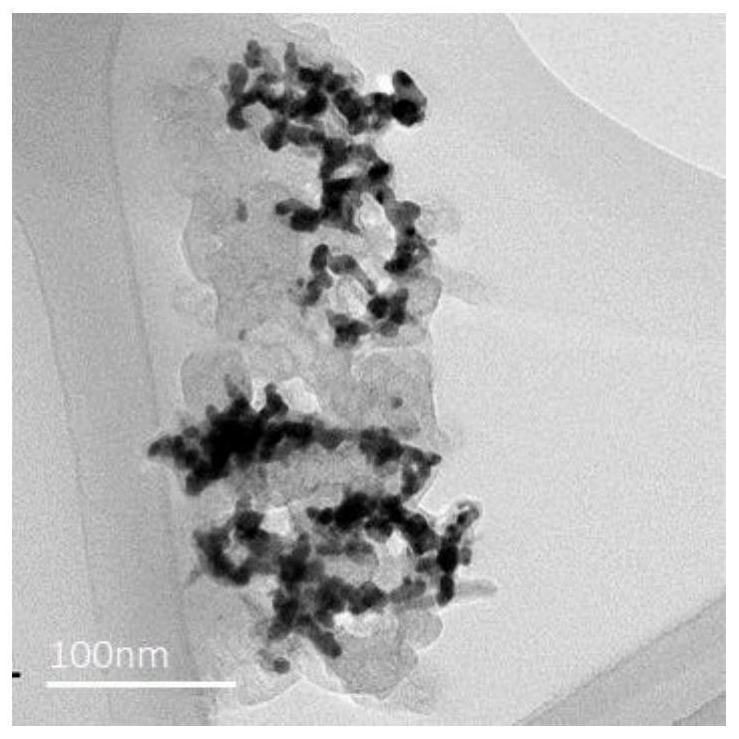
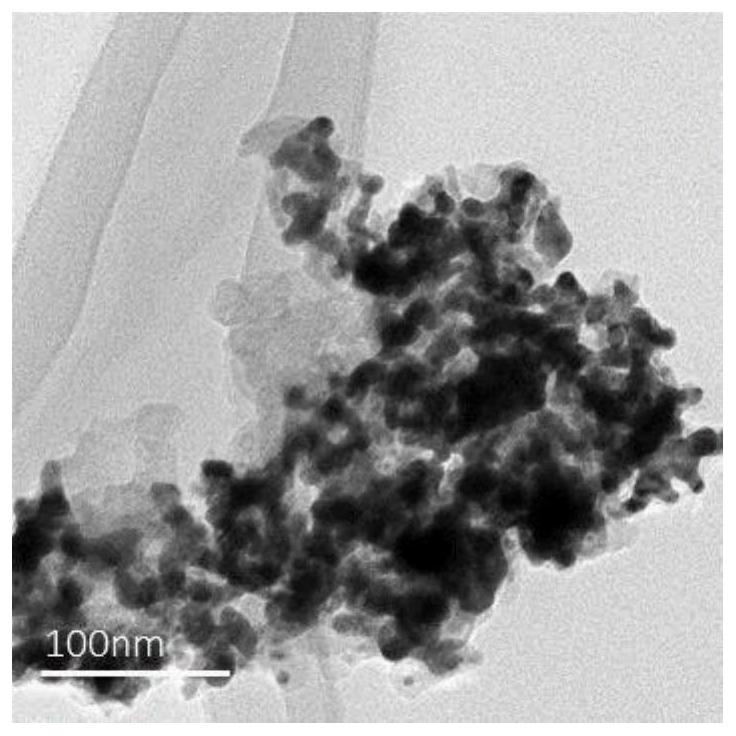
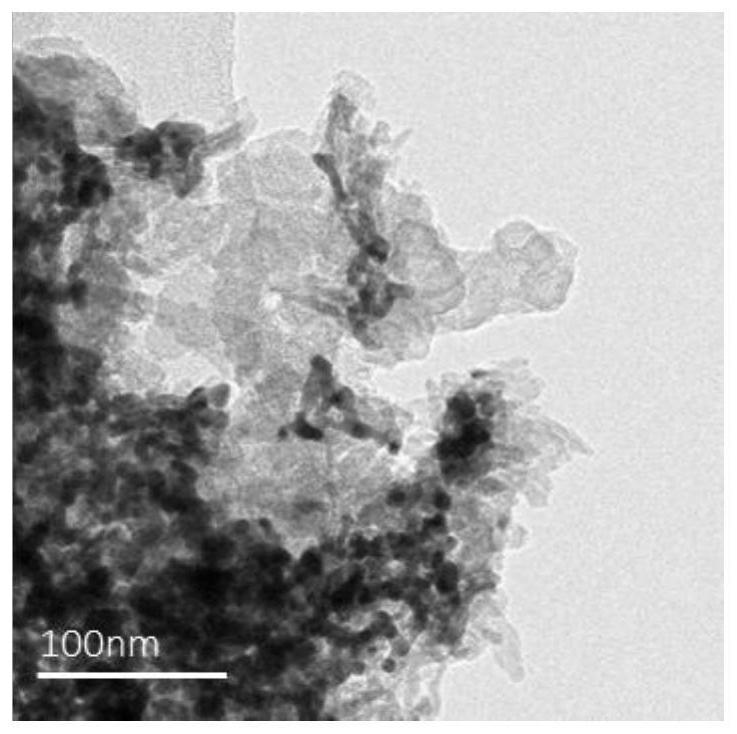
Image
Examples
Embodiment 1
[0045] Step 1: Accurately weigh 23.8 mg of copper chloride dihydrate and dissolve in 50 mL of water to form precursor solution A; weigh 21.5 mg of chloroauric acid and dissolve in 50 mL of water to form precursor solution B.
[0046] Step 2: Under stirring, add 200 μL of PDDA dropwise to the precursor solution A in step 1 to form solution C; under stirring, add 750 μL of 0.1M sodium citrate solution dropwise to the precursor in step 1 In solution B, solution D was formed; 47 mg of sodium borohydride was weighed and dissolved in 15 mL of ice water to form solution E, and 4.6 mg of sodium borohydride was weighed and dissolved in 15 mL of ice water to form solution F.
[0047] Step 3: Add solution E in step 2 dropwise to solution C in step 2 under Ar atmosphere and stirring in an ice bath, stir for 2 hours to form solution G, and add solution F in step 2 to solution D dropwise , stirred for 2 h to form solution H.
[0048] Step 4: After the stirring is finished, add solution G i...
Embodiment 2
[0054] Step 1: Accurately weigh 47.6 mg of copper chloride dihydrate and dissolve in 50 mL of water to form precursor solution A; weigh 43.3 mg of chloroauric acid and dissolve in 50 mL of water to form precursor solution B.
[0055] Step 2: Under stirring, add 200 μL of PDDA solution dropwise to precursor solution A in step 1 to form solution C; under stirring, add 750 μL of 0.1M sodium citrate solution dropwise to the precursor in step 1 94 mg of sodium borohydride was dissolved in 15 mL of ice water to form solution E, and 9.2 mg of sodium borohydride was weighed and dissolved in 15 mL of ice water to form solution F.
[0056] Step 3: Under the conditions of Ar atmosphere and ice bath stirring, add solution E in step 2 dropwise to solution C in step 2, stir for 2 hours to form solution G, and add solution F in step 2 dropwise to solution D1 , stirred for 2 h to form solution H.
[0057] Step 4: After the stirring is finished, add solution G in step 3 dropwise to solution H...
Embodiment 3
[0063] Step 1: Accurately weigh 71.5 mg of copper chloride dihydrate and dissolve in 50 mL of water to form precursor solution A; weigh 64.9 mg of chloroauric acid and dissolve in 50 mL of water to form precursor solution B.
[0064] Step 2: Under stirring, add 200 μL of PDDA solution dropwise to precursor solution A in step 1 to form solution C; under stirring, add 750 μL of 0.1M sodium citrate solution dropwise to the precursor in step 1 141.8 mg sodium borohydride was weighed and dissolved in 15 mL ice water to form solution E, and 46.1 mg was weighed and dissolved in 15 mL ice water to form solution F.
[0065] Step 3: Add solution E in step 2 dropwise to solution C in step 2 under Ar atmosphere and stirring in an ice bath, stir for 2 hours to form solution G, and add solution F in step 2 to solution D dropwise , stirred for 2 h to form solution H.
[0066] Step 4: After the stirring is finished, add solution G in step 3 dropwise to solution H in step 3, and stir for 2 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com