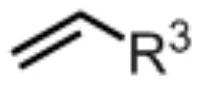
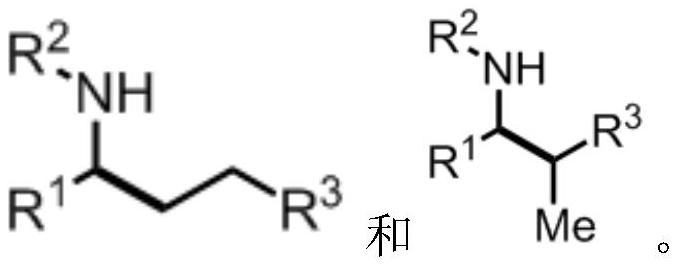
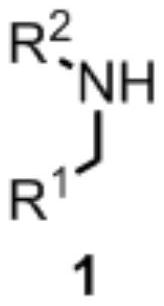
Method for synthesizing alkylamine derivative
A derivative, alkylamine technology, applied in the field of dehydrogenation reduction coupling reaction, can solve problems such as narrow applicability, poor atom economy, excess, etc., and achieve the effects of simple operation, avoidance of subsequent processing, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of N-(1,3-Diphenylpropyl)-4-methylbenzenesulfonamide
[0038]
[0039] In a nitrogen atmosphere, sequentially add raw material 1a (0.2mmol, 52.2mg), raw material 2a (0.4mmol, 41.6mg), KO t Bu(0.05mmol, 5.6mg), PhB(OH) 2 (0.05mmol, 6.1mg), Ni(cod) 2 (0.005mmol, 1.4mg), and PCy 3 (0.01mmol, 2.8mg), finally added Toluene (0.3mL), stirred at 120°C for 20 hours, cooled to room temperature, concentrated, and directly separated by column chromatography to obtain the target product 3a with a yield of 93%, 67.8mg. 1 H NMR (500MHz, CDCl 3 )δ7.54(d,J=8.0Hz,2H),7.20–7.17(m,2H),7.14–7.08(m,4H),7.03–6.99(m,6H),6.01(d,J=8.0Hz ,1H),4.28(dd,J 1 =15.0Hz,J 2 =7.5Hz,1H),2.58–2.52(m,1H),2.49–2.43(m,1H),2.29(s,3H),2.12–2.05(m,1H),1.99–1.92(m,1H); 13 C NMR (125MHz, CDCl 3 )δ142.7,140.8,140.6,137.5,129.1,128.24,128.19,127.1,126.9,126.5,125.8,57.8,38.9,32.0,21.3; HRMS(ESI)m / z calcd.For C 22 h 23 NNaO 2 S[M+Na] +:388.1342,found:388.1345.
[0040] Scaled-up preparation...
Embodiment 2
[0043] Preparation of N-(1,3-Diphenylpropyl)methanesulfonamide
[0044]
[0045] In a nitrogen atmosphere, sequentially add raw material 1b (0.2mmol, 37.0mg), raw material 2a (0.4mmol, 41.6mg), KO t Bu(0.05mmol, 5.6mg), PhB(OH) 2 (0.05mmol, 6.1mg), Ni(cod) 2 (0.005mmol, 1.4mg), and PCy 3 (0.01mmol, 2.8mg), finally added Toluene (0.3mL), stirred at 120°C for 20 hours, cooled to room temperature, concentrated, and directly separated by column chromatography to obtain the target product 3b with a yield of 96%, 55.5mg. 1 H NMR (500MHz, CDCl 3 )δ7.40–7.37(m,2H),7.33–7.25(m,2H),7.19–7.17(m,1H),7.14(d,J=7.0Hz,2H),5.18–5.15(m,1H) ,4.46(dd,J 1 =15.0Hz,J 2 =7.5Hz,1H),2.71–2.65(m,1H),2.61–2.55(m,1H),2.53(s,3H),2.21–2.13(m,1H),2.10–2.03(m,1H); 13 C NMR (125MHz, CDCl 3 )δ141.2, 140.7, 129.0, 128.5, 128.4, 128.1, 126.7, 126.1, 57.9, 41.8, 39.2, 32.3.
Embodiment 3
[0047] Preparation of N-(1,3-Diphenylpropyl)-2,4,6-trimethylbenzonesulfonamide
[0048]
[0049] In an argon atmosphere, sequentially add raw material 1c (0.2mmol, 57.8mg), raw material 2a (0.4mmol, 41.6mg), KO t Bu(0.05mmol, 5.6mg), PhB(OH) 2 (0.05mmol, 6.1mg), Ni(cod) 2 (0.005mmol, 1.4mg), and PCy 3 (0.01mmol, 2.8mg), finally added Toluene (0.3mL), stirred at 120°C for 20 hours, cooled to room temperature, concentrated, and directly separated by column chromatography to obtain the target product 3c with a yield of 98%, 77.0mg. 1 H NMR (500MHz, CDCl 3 )δ7.24–7.21(m,2H),7.17–7.11(m,4H),7.02(d,J=7.5Hz,2H),6.96–6.94(m,2H),6.77(s,2H),5.01 –4.98(m,1H),4.18(dd,J 1 =14.0Hz,J 2 =7.0Hz,1H),2.55–2.44(m,8H),2.23(s,3H),2.16–2.09(m,1H),2.05–1.98(m,1H); 13 C NMR (125MHz, CDCl 3 )δ141.9, 140.8, 140.3, 138.7, 134.3, 131.7, 128.37, 128.35, 128.32, 127.5, 126.3, 126.0, 57.6, 38.6, 32.0, 22.8, 20.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com