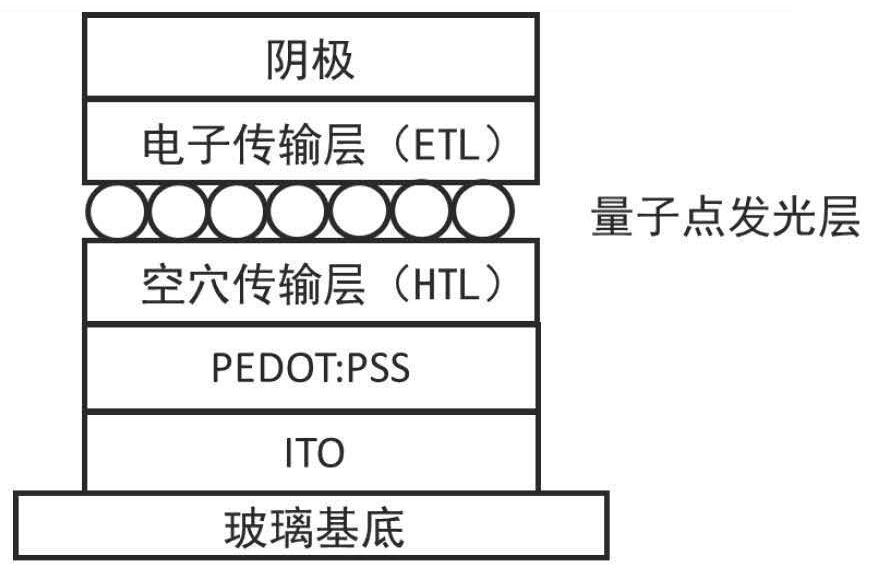
Organic small molecule electron transport material based on naphthalimide unit and application thereof
An electron transport material, a technology of naphthalene diimide, applied in the field of organic small molecule electron transport materials, can solve the problems of insufficient device efficiency and stability, high LUMO energy level, unfavorable electron injection and transport in devices, etc. Improve electroluminescence efficiency, improve stability, and facilitate processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: DNDI (4-b-C8) small organic molecule, structural formula is as follows:
[0048]
[0049] The preparation method is: weigh 1,4,5,8-naphthalene tetracarboxylic anhydride (2.68g, 10mmol); put 25mL concentrated sulfuric acid in a 100mL single-necked bottle, stir for 1h until the system is clear, add brominated reagent 1,3, 5-Tribromo-1,3,5-thiazinane-2,4,6-trione (1.83g, 5mmol) was reacted at room temperature for 8h. After the reaction, the system was poured into ice water, stirred for 3 h, filtered with suction, washed with methanol and dried to obtain the crude product as light yellow powder. The product was recrystallized from N,N-dimethylformamide to obtain white crystals of bromonaphthalic anhydride (Br-NDA). Yield 2.42g, 70% yield.
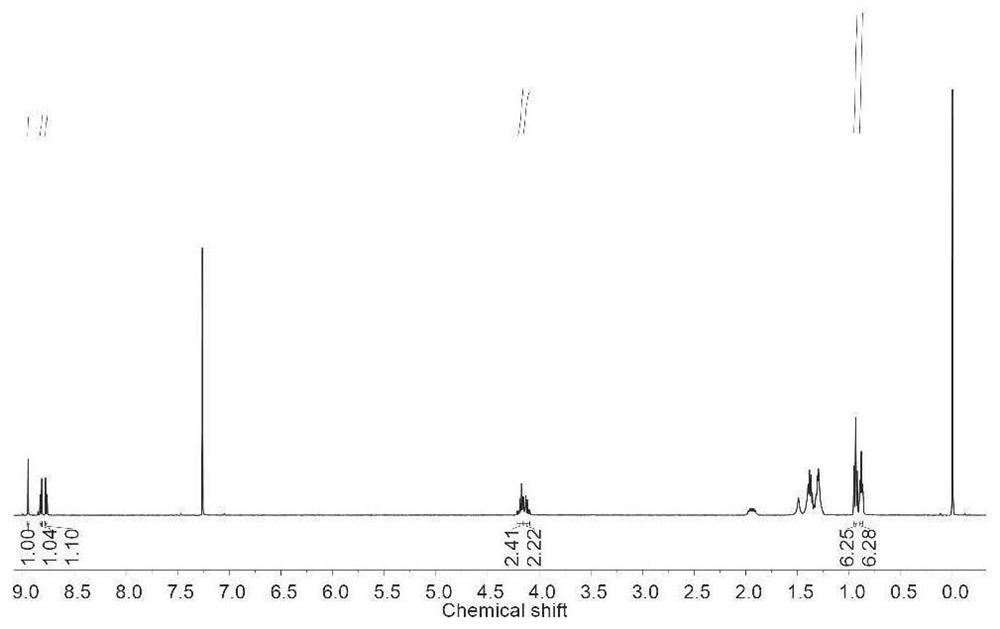
[0050] Carry out nuclear magnetic analysis to the monomer of preparation, the result is as follows:1 H NMR (400MHz, DMSO-d 6 ):δ8.71(s,1H),8.57(d,1H),8.21(d,1H); 13 C NMR (100MHz, DMSO-d 6 ): δ168.1, 160.0, 159.4, 1...
Embodiment 2
[0059] Embodiment 2: DNDI (4-d-C5) small organic molecule, structural formula is as follows:
[0060]
[0061] The preparation method is: weigh 1,4,5,8-naphthalene tetracarboxylic anhydride (2.68g, 10mmol); put 25mL concentrated sulfuric acid in a 100mL single-necked bottle, stir for 1h until the system is clear, add brominated reagent 1,3, 5-Tribromo-1,3,5-thiazinane-2,4,6-trione (1.83g, 5mmol) was reacted at room temperature for 8h. After the reaction, the system was poured into ice water, stirred for 3 h, filtered with suction, washed with methanol and dried to obtain the crude product as light yellow powder. The product was recrystallized from N,N-dimethylformamide to obtain white crystals of bromonaphthalic anhydride (Br-NDA). Yield 2.42g, 70% yield.
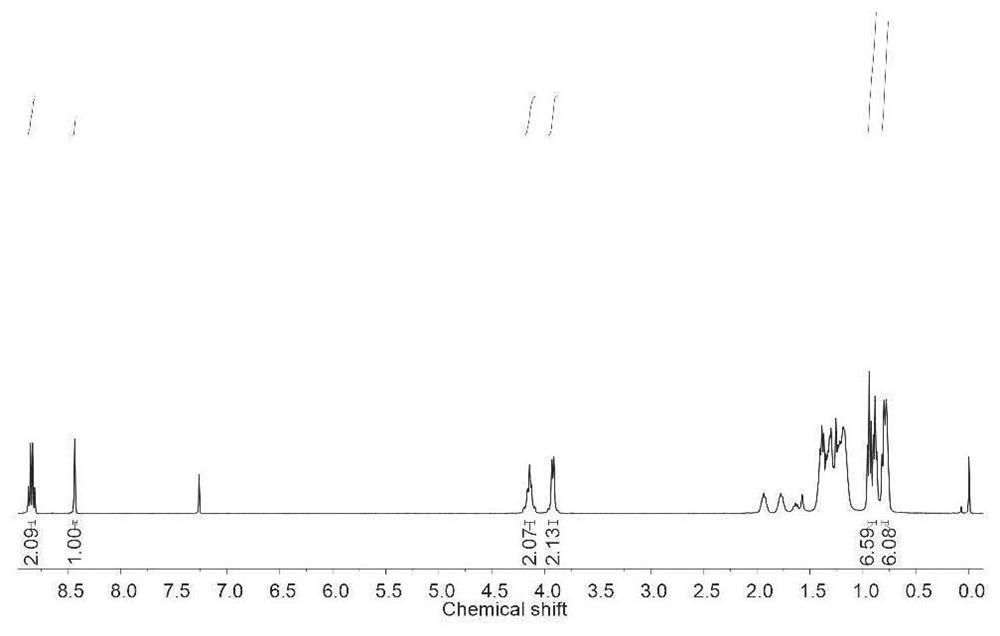
[0062] Carry out nuclear magnetic analysis to the monomer of preparation, the result is as follows: 1 H NMR (400MHz, DMSO-d 6 ):δ8.71(s,1H),8.57(d,1H),8.21(d,1H); 13 C NMR (100MHz, DMSO-d 6 ): δ168.1, 160.0, 159.4,...
Embodiment 3
[0071] Embodiment 3: DNDI (4-n-C6) small organic molecule, structural formula is as follows:
[0072]
[0073] The preparation method is: weigh 1,4,5,8-naphthalene tetracarboxylic anhydride (2.68g, 10mmol); put 25mL concentrated sulfuric acid in a 100mL single-necked bottle, stir for 1h until the system is clear, add brominated reagent 1,3, 5-Tribromo-1,3,5-thiazinane-2,4,6-trione (1.83g, 5mmol) was reacted at room temperature for 8h. After the reaction, the system was poured into ice water, stirred for 3 h, filtered with suction, washed with methanol and dried to obtain the crude product as light yellow powder. The product was recrystallized from N,N-dimethylformamide to obtain white crystals of bromonaphthalic anhydride (Br-NDA). Yield 2.42g, 70% yield.
[0074] Carry out nuclear magnetic analysis to the monomer of preparation, the result is as follows: 1 H NMR (400MHz, DMSO-d 6 ):δ8.71(s,1H),8.57(d,1H),8.21(d,1H); 13 C NMR (100MHz, DMSO-d 6 ): δ168.1, 160.0, 159.4,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com