Method for preparing ammonium manganese phosphate crystal
A technology for ammonium manganese phosphate and crystals, which is applied in the field of bionic method for preparing ammonium manganese phosphate crystals, can solve the problems of high energy consumption, complicated process and high cost of ammonium manganese phosphate, and achieves the advantages of simple operation, low preparation cost, mild and safe reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
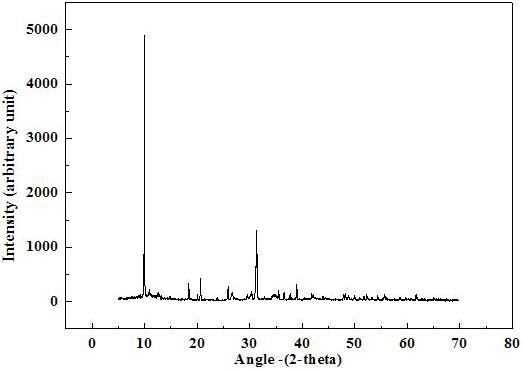
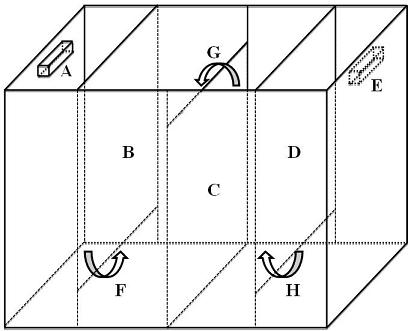
[0013] In a connected reactor with 2 left and right ear grooves and 3 left, middle and right barrier plates, according to the molar ratio, manganese: phosphorus = 1: 1.50, manganese chloride is added to the left ear groove, and ammonium phosphate is added to the right ear in the slot. Then, a mixture solution containing 0.04% starch and 2.0% ammonium chloride by mass ratio is added to the connected reactor, so that the liquid level of the mixture solution of starch and ammonium chloride is higher than the left ear groove and the right ear groove The upper edge is 1 cm to ensure that the reactants in the left ear groove and the right ear groove are connected through the mixture solution. Finally, the lid was put on the connected reactor, and after 7 days of static reaction, the product was filtered, washed, and dried at 105° C. for 1 hour to obtain ammonium manganese phosphate crystals. The yield of ammonium manganese phosphate crystals was 98%, and the purity was 99.5%.
Embodiment 2
[0015] In a connected reactor with 2 left and right ear grooves and 3 left, middle and right barrier plates, according to the molar ratio, manganese: phosphorus = 1: 1.40, manganese chloride is added to the left ear groove, and ammonium phosphate is added to the right ear in the slot. Then, a mixture solution containing 0.03% starch and 2.5% ammonium chloride by mass ratio is added to the connected reactor, so that the liquid level of the mixture solution of starch and ammonium chloride is higher than the left ear groove and the right ear groove The upper edge is 1 cm to ensure that the reactants in the left ear groove and the right ear groove are connected through the mixture solution. Finally, the lid was put on the connected reactor, and after 7 days of static reaction, the product was filtered, washed, and dried at 105° C. for 1 hour to obtain ammonium manganese phosphate crystals. The yield of ammonium manganese phosphate crystals was 97%, and the purity was 99.9%.
Embodiment 3
[0017] In a connected reactor with 2 left and right ear grooves and 3 left, middle and right barrier plates, according to the molar ratio, manganese: phosphorus = 1: 1.60, manganese chloride is added to the left ear groove, and ammonium phosphate is added to the right ear in the slot. Then, a mixture solution containing 0.05% starch and 1.5% ammonium chloride by mass ratio is added to the connected reactor, so that the liquid level of the mixture solution of starch and ammonium chloride is higher than the left ear groove and the right ear groove The upper edge is 1 cm to ensure that the reactants in the left ear groove and the right ear groove are connected through the mixture solution. Finally, the lid was put on the connected reactor, and after 7 days of static reaction, the product was filtered, washed, and dried at 105° C. for 1 hour to obtain ammonium manganese phosphate crystals. The yield of ammonium manganese phosphate crystals was 99%, and the purity was 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com