Synthesis method and application of pefloxacin mesylate
A technology of pefloxacin mesylate and its synthesis method, which is applied to the preparation of sulfonate, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve problems such as operator danger and equipment corrosion, and reduce the process flow , reduce corrosion, improve the effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
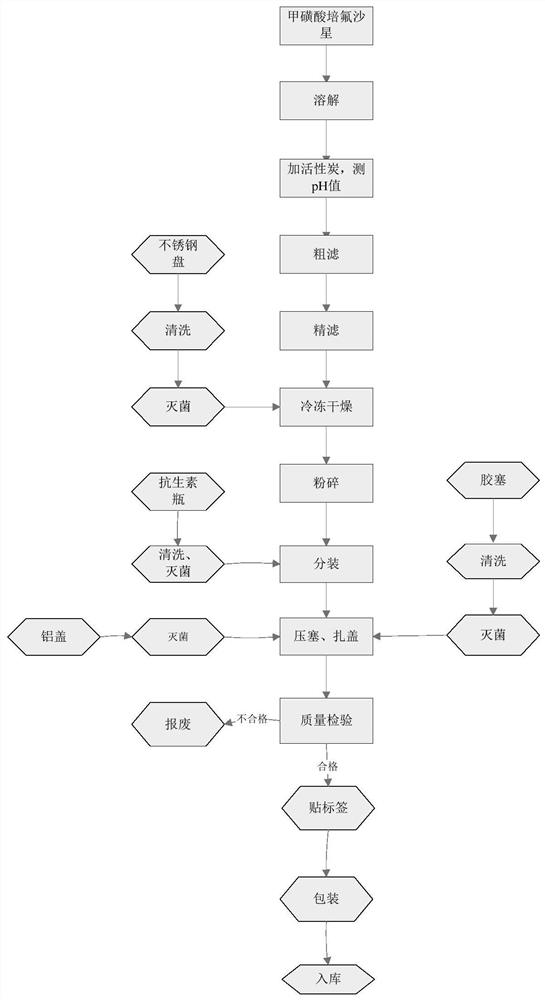
Image
Examples
Embodiment 1
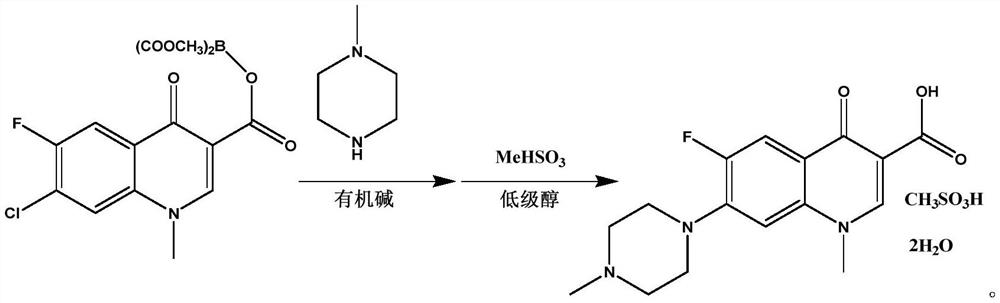
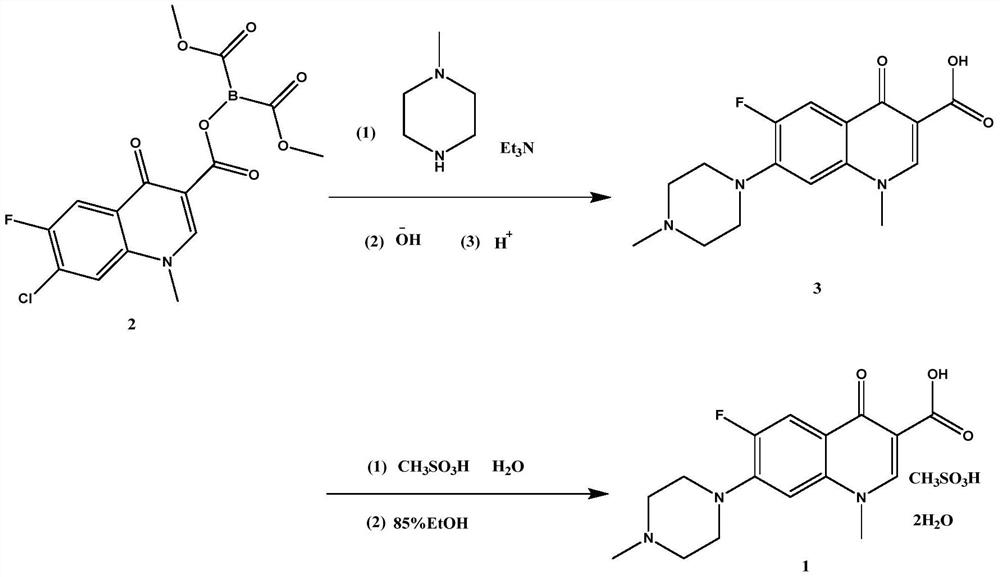
[0027] Embodiment 1 A kind of synthetic method of pefloxacin mesylate A1
[0028] The present embodiment provides a kind of synthetic method of pefloxacin mesylate A1, it is
[0029] Weigh 38.3g dimethyl ((7-chloro-6-fluoro-1-methyl-4-carbonyl-1,4-dihydroquinoline-3-carbonyl) oxo) borane dicarboxylate and 10.0 Add g N-methylpiperazine to 50ml THF and stir, add 42ml TEA while stirring continuously, raise the temperature to 60°C and keep stirring for 4 hours, take a small amount of reaction solution and send it to liquid chromatography to monitor the reaction progress, the peak of the raw material disappears, which is the end of the reaction , Evaporate the residual solvent and TEA under reduced pressure, add 100ml ethanol to mix and dissolve, add 28.5g methanesulfonic acid, heat up to 90°C and continue stirring for 6h, a large amount of solid precipitates, TLC monitors the reaction, and the disappearance of the raw material point is the end of the reaction. Filter the reaction...
Embodiment 2~6
[0032] The synthetic method of embodiment 2~6 pefloxacin mesylate A2~A6
[0033] The synthesis method of pefloxacin mesylate A2-A6 provided in Examples 2-6 is basically the same as the synthesis method of pefloxacin mesylate A1 described in Example 1, the difference is that some process parameters are different, specifically The process parameters are shown in Table 1.
[0034] Table 1: Process parameter table of pefloxacin mesylate A2~A6
[0035]
[0036]
[0037] Other parameters are all the same as in Example 1.
experiment example
[0042] Experimental example Stability test of pefloxacin mesylate
[0043] Choose pefloxacin mesylate A1~A6 provided by the present invention, pefloxacin mesylate D and pefloxacin mesylate S (Rewell Biotechnology) to carry out stability comparative research test, detection standard With the standard described in "Chinese Pharmacopoeia" Part Two (p228):
[0044] Selected sample is placed 3 months under the condition of temperature 40 ℃ ± 2 ℃, relative humidity 75% ± 5%, through sampling analysis, the comparison of each index analysis result and 0 month, methanesulfonic acid cultivation provided by the present invention The related substances of flufloxacin A1~A6 increased significantly, and all other detection indexes had obvious changes. % ± 5% under the condition of instability, so the accelerated test of this condition is no longer carried out.
[0045] The selected samples were stored for 6 months at a temperature of 30°C ± 2°C and a relative humidity of 65% ± 5%. After s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com