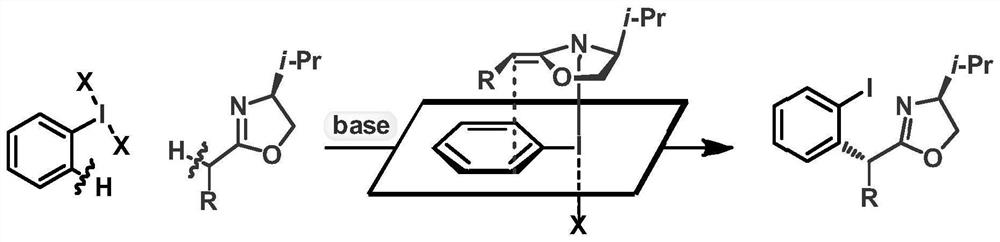
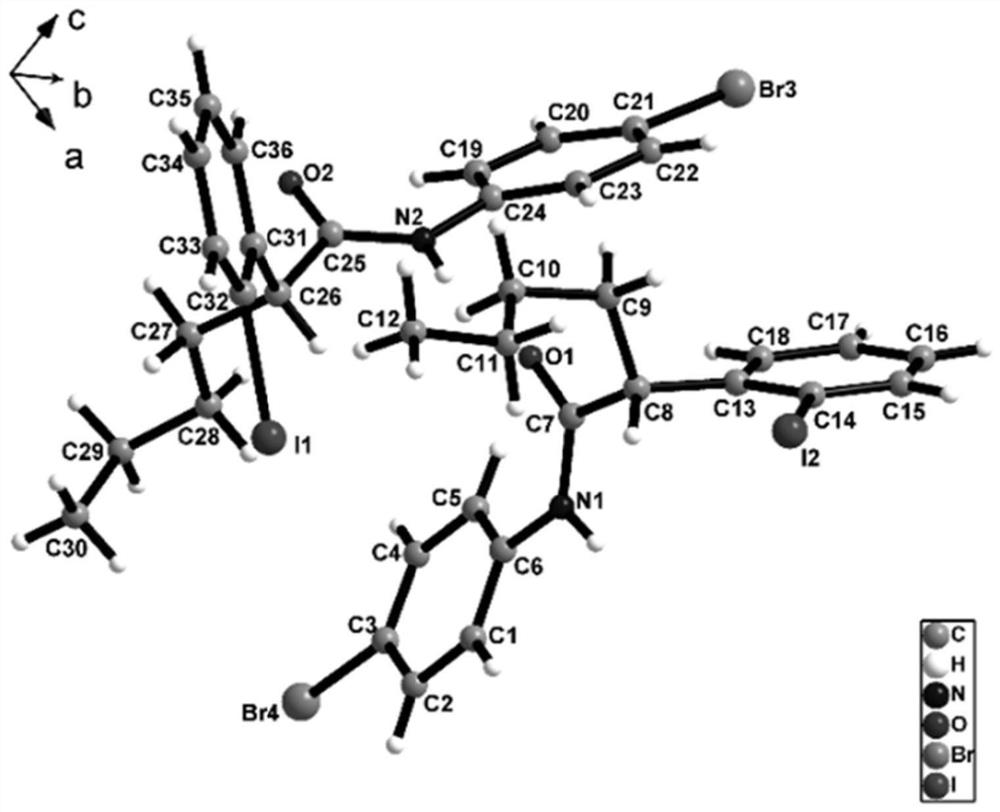
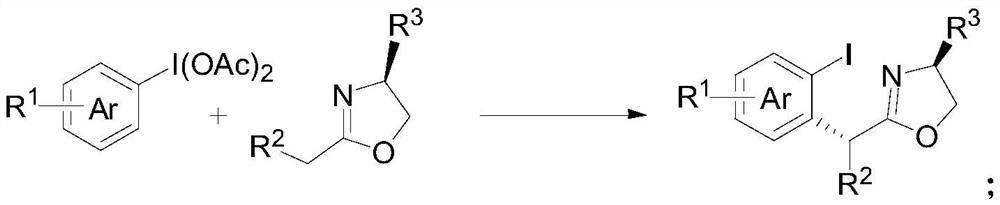
Method for preparing chiral alpha-arylcarbonyl compound and product
An aryl carbonyl and compound technology, which is applied in the field of organic chemical synthesis, can solve the problems of complicated steps and difficult to obtain raw materials, and achieves the effect of cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The specific implementation examples of the new method for preparing chiral α-arylcarbonyl compounds provided by the present invention are introduced below, and multiple specific implementation examples are provided. It should be noted that the implementation of the present invention is not limited to the following implementation examples.
[0048] In the embodiment, the raw material (II) adopts commercially available products, and the raw material (III) is partially derived from commercially available products, and the preparation method of some compounds is as follows:
[0049] (S)-4-isobutyl-2-pentyl-4,5-dihydrooxazole (6a):
[0050] 0 ℃ to (S)-2-amino-3-methylbutyl-1-yl alcohol (2.48g, 24mmol) and Et 3 To a solution of N (3.3 mL, 24 mmol) in DCM (67 mL), hexanoyl chloride (2.8 mL, 20 mmol) was added dropwise. The mixture was warmed to room temperature, stirred for 3 h, DCM (33 mL) and Et 3 N (13.9 mL, 100 mmol). MsCl (1.9 mL, 24 mmol) was added at 0° C. and stir...
Embodiment 1
[0062] R 1 for H, R 2 is n-butyl, R 3 For isopropyl group, 238.9 mg of yellow liquid compound 1 was obtained by the above method, and the yield was 62% (>20 / 1dr). 1 H NMR (600MHz, CDCl 3 ):δ7.85–7.81(m,1H),7.44–7.40(m,1H),7.33–7.27(m,1H),6.95–6.89(m,1H),4.21–4.16(m,1H),4.02 (t,J=7.6Hz,1H),3.98–3.93(m,1H),3.89(t,J=7.9Hz,1H),2.07–1.96(m,1H),1.85–1.79(m,2H), 1.37–1.30(m,3H),1.24–1.18(m,1H),0.93(d,J=6.8Hz,3H),0.88–0.83(m,6H).
[0063] 13 C NMR (151MHz, CDCl 3 ): δ167.9, 143.5, 139.7, 128.7, 128.6, 128.0, 102.0, 71.9, 69.5, 49.0, 34.2, 32.4, 29.6, 22.7, 19.1, 17.8, 14.1.
[0064] IR(neat):2955,2928,2870,1661,1465,1435,1106,745.
[0065] HRMS (ESI-TOF): calculated for [C 17 h 25 INO(M+H + )]:386.0975,found:386.0976.
Embodiment 2
[0067] R 1 for H, R 2 is isopentyl, R 3 For isopropyl group, 231.6 mg of yellow liquid compound 2 (>20 / 1dr) can be obtained by the same method, and the yield is 58%. 1 H NMR (400MHz, CDCl 3 ):δ7.85–7.80(m,1H),7.44–7.40(m,1H),7.32–7.27(m,1H),6.94–6.88(m,1H),4.21–4.16(m,1H),4.00 (t,J=7.6Hz,1H),3.98–3.93(m,1H),3.90(t,J=7.9Hz,1H),2.04–1.96(m,1H),1.87–1.78(m,2H), 1.60–1.51(m,1H),1.30–1.22(m,1H),1.15–1.05(m,1H),0.93(d,J=6.8Hz,3H),0.88–0.82(m,9H).
[0068] 13 C NMR (101MHz, CDCl 3 ): δ168.0, 143.4, 139.7, 128.7, 128.6, 128.0, 102.0, 71.7, 69.5, 49.2, 36.4, 32.27, 32.25, 28.0, 22.7, 22.6, 19.1, 17.7.
[0069] IR(neat):2953,2899,2869,1662,1465,1384,1365,1170,1010,742.
[0070] HRMS (ESI-TOF): calculated for [C 18 h 27 INO((M+H + )]:400.1132,found:400.1129.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com