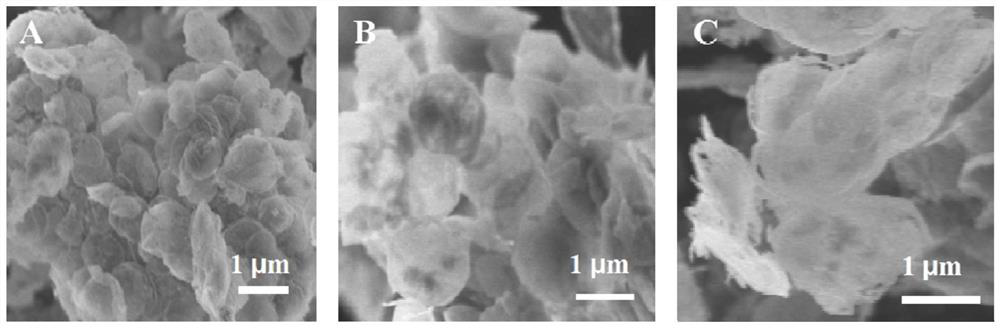
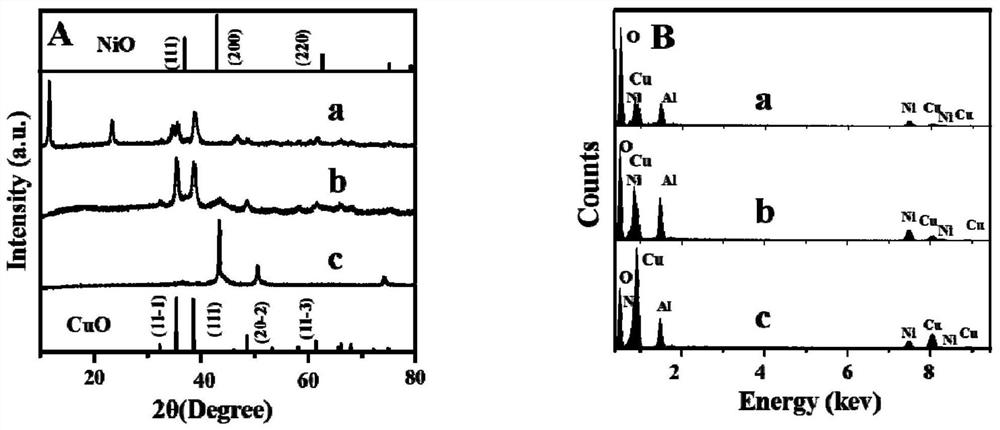
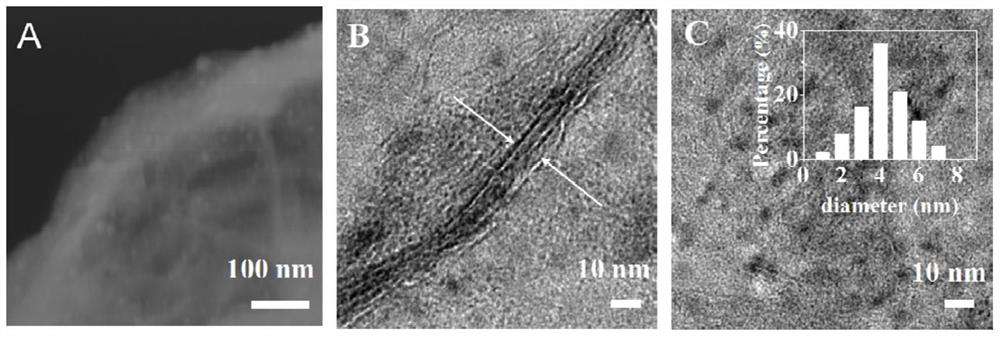
Preparation method and application of CuNi-Cu2O/NiAlOx nano composite material with two-dimensional layered structure
A nanocomposite material, cuni-cu2o technology, applied in the field of photocatalysis, can solve problems such as limiting effectiveness, and achieve the effect of convenient and simple product processing, abundant reserves, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] (1) Preparation of CuNiAl-LDH
[0074] In the first step, weigh 426.2mg of copper chloride dihydrate and dissolve it in a 250mL volumetric flask. Transfer 7.5ml with a pipette gun to a polytetrafluoroethylene reaction kettle;
[0075] In the second step, take 594.2 mg of nickel chloride hexahydrate and dissolve it in a 250 mL volumetric flask. Transfer 3.3ml with a pipette gun to a polytetrafluoroethylene reaction kettle;
[0076] In the third step, weigh 603.6 mg of aluminum chloride hexahydrate and dissolve it in a 250 mL volumetric flask. Transfer 2.7ml with a pipette gun to a polytetrafluoroethylene reaction kettle;
[0077] In the fourth step, weigh 1.501 g of urea and dissolve it in a 250 mL volumetric flask. Pipette 7.5ml and transfer it to a polytetrafluoroethylene reaction kettle;
[0078] The fifth step is to place the reaction kettle in an electric constant temperature blast drying oven, raise the temperature from room temperature to 140°C at a rate of 2...
Embodiment 2
[0097] (1) Preparation of CuNiAl-LDH
[0098] In the first step, weigh 456.4mg of copper acetate monohydrate and dissolve it in a 250mL volumetric flask. Transfer 7.5ml to a three-neck round bottom flask with a pipette;
[0099] In the second step, weigh 534.8 mg of nickel nitrate hexahydrate and dissolve it in a 250 mL volumetric flask. Transfer 3ml to a three-neck round bottom flask with a pipette gun;
[0100] In the third step, weigh 593.2 mg of aluminum nitrate nonahydrate and dissolve it in a 250 mL volumetric flask. Transfer 2.5ml to a three-neck round bottom flask with a pipette;
[0101] In the fourth step, weigh 1.83g of urea and dissolve it in a 250mL volumetric flask. Transfer 7ml to a three-neck round bottom flask with a pipette gun;
[0102] Step 5: Place the three-necked round-bottomed flask in an oil bath, raise the temperature from room temperature to 90°C at a rate of 2°C / min and keep it warm for 15 hours;
[0103] The sixth step, wait until the reactio...
Embodiment 3
[0109] (1) Preparation of CuNiAl-LDH
[0110] In the first step, weigh 91.8 mg of copper acetate monohydrate and dissolve it in a 250 mL volumetric flask. Transfer 6.5ml with a pipette gun to a polytetrafluoroethylene reaction kettle;
[0111] In the second step, weigh 544.2 mg of nickel acetate tetrahydrate and dissolve it in a 250 mL volumetric flask. Use a pipette gun to transfer 3ml to a polytetrafluoroethylene reaction kettle;
[0112] In the third step, weigh 589.5 mg of aluminum nitrate nonahydrate and dissolve it in a 250 mL volumetric flask. Use a pipette gun to transfer 3ml to a polytetrafluoroethylene reaction kettle;
[0113] In the fourth step, weigh 0.391 g of urea and dissolve it in a 250 mL volumetric flask. Pipette 7.5ml and transfer it to a polytetrafluoroethylene reaction kettle;
[0114] The fifth step is to place the reaction kettle in an electric constant temperature blast drying oven, raise the temperature from room temperature to 160°C at a rate of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com