Zn/H2aip fluorescent probe, preparation and application of Zn/H2aip fluorescent probe in detection of tetracycline antibiotics
A fluorescent probe, tetracycline technology, used in the application field of identifying tetracycline antibiotics by fluorescence integration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Synthesis of fluorescent probes
[0030] Take ZnO (0.05g), 5-aminoisophthalic acid (0.1g) and 8mL of DMF, treat the mixture with ultrasonic, and put it in a reaction kettle at 120°C for 48 hours. After taking it out, it was washed successively with DMF and absolute ethanol, dried in a vacuum oven at 60 °C and then ground to obtain Zn / H 2 aip fluorescent probe. The probe material is prepared as a 1mg / mL stock solution, which can be stored in the refrigerator for future use.
[0031] (2) Qualitative and quantitative detection of TCs
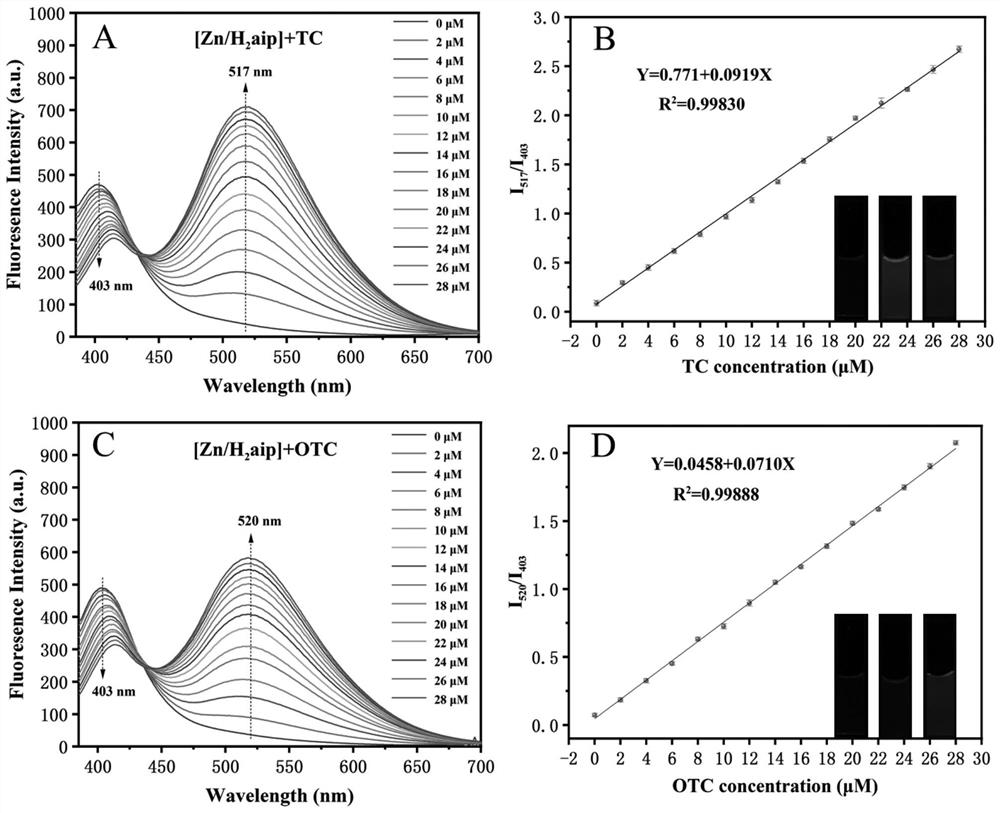
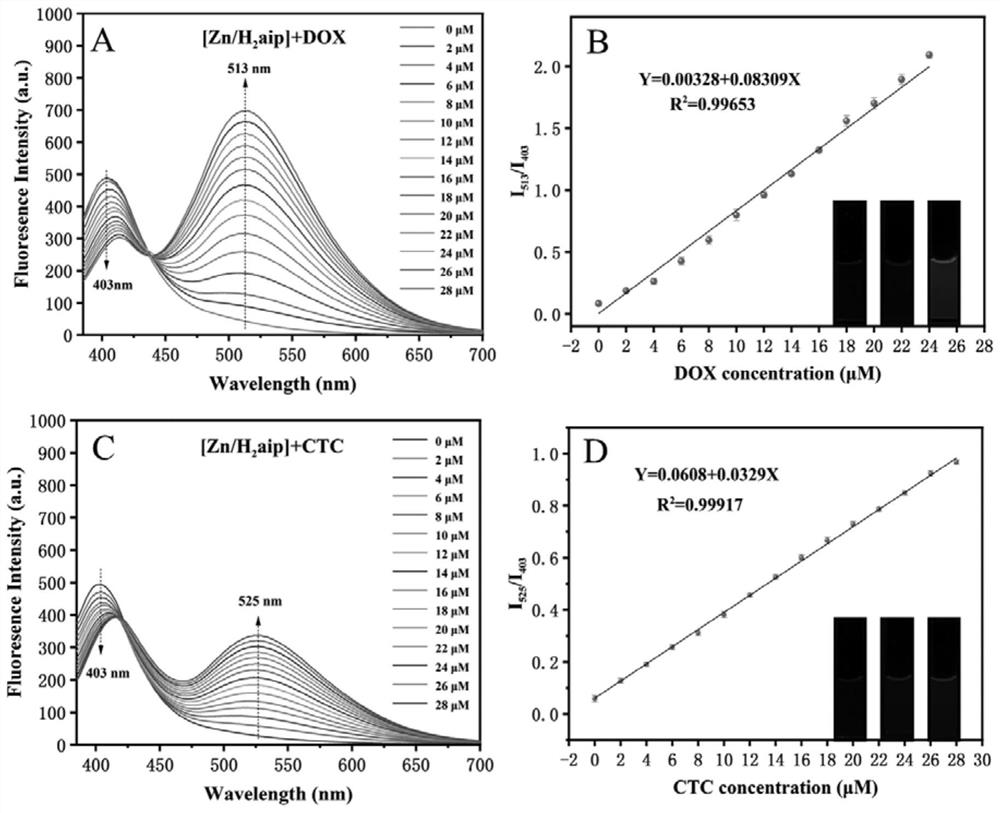
[0032] Probe Zn / H of the present invention 2 The fluorescence response of aip to TCs was carried out in Tris-HCl buffer (10 mM, pH=8). 100 μL Probe Zn / H 2 aip (1mg / mL) was added to a cuvette containing 2mL of buffer, and it was incubated for 30 minutes. The final concentration of the probe was 0.05mg / mL, and then TCs were added to the above solution. The final concentrations of TCs were 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24...
Embodiment 2
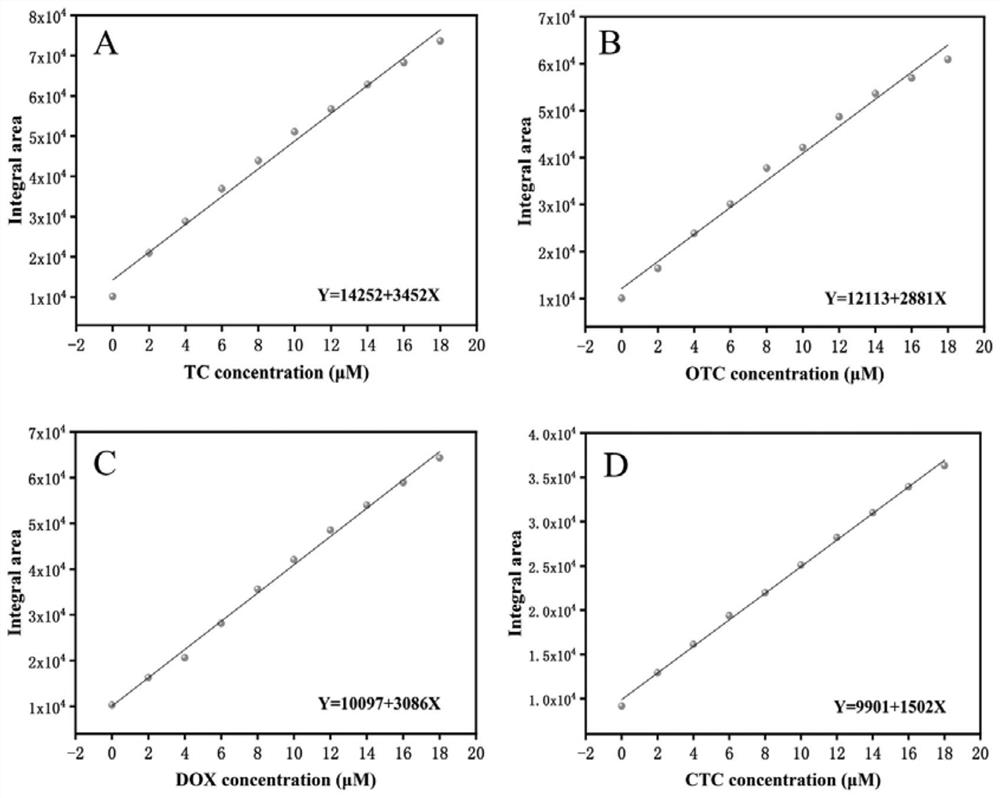
[0040] The doxycycline sample solution was prepared and placed in 2 mL of Tris-HCl buffer solution (10 mM, pH=8) with a final concentration of 4 μM. The integral area method of the present invention is adopted for detection. First 100 μL probe Zn / H 2 aip (1mg / mL) was added to the cuvette of the above-mentioned 2mL Tris-HCl buffer, and incubated for 30 minutes. The final concentration of the probe was 0.05mg / mL, and the fluorescence detection was carried out with 365nm as the excitation wavelength. The fluorescence spectrum was from 385nm is recorded to 700nm, and the area under the curve between 436nm and 700nm is integrated to obtain the area integral value, and then according to image 3 The readings of the four standard curves are calculated to obtain a concentration range of 4.01-12.12 μM in the TCs sample solution. Through the application "TCs Analysis", the interface can directly display "TCs contamination degree: 4.01-12.12μM".
Embodiment 3
[0042] The doxycycline sample solution was prepared so that it was placed in 2 mL Tris-HCl buffer solution with a final concentration of 8 μM. The method described in Example 2 was used for detection, and the concentration of the TCs sample solution was obtained to be 8.05-21.4 μM. The application "TCs Analysis" can directly display "TCs contamination degree: 8.05-21.4μM" on the interface.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com