Synthetic method of triamterene intermediate
A technology of triamterene and a synthesis method, applied in the field of medicine, can solve the problems of high cost of triaminopyrimidine, low equipment return, high environmental pressure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 triamterene intermediate
[0025] 1. Add 340.2g of pure water and 134.5g of propanedinitrile to the reaction kettle in sequence. After stirring for 25 minutes to dissolve, turn on the nitrogen protection, add 485.7 aqueous solution containing 162.1g of sodium nitrite at 15°C, and add 8% HCl dropwise to adjust the pH3 ~4. After the sodium nitrite aqueous solution is added dropwise, 70.4g of dilute hydrochloric acid is consumed, and kept at 18°C for 2.5h;
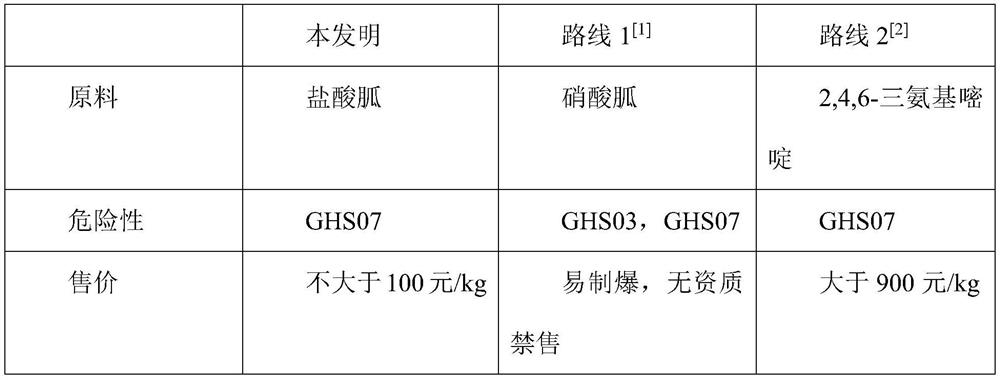
[0026] 2. Add 226.3g of guanidine hydrochloride solid to 1, stir for 30min; add 17.6g of NaCO3 solid to adjust the pH to 9-10;
[0027] 3. Add 1340ml of a mixed solvent of dimethyl sulfoxide and xylene, heat up to reflux with water, keep warm at 140°C for 2 hours, and prepare for filtration; the composition of the mixed solvent: dimethyl sulfoxide: xylene is 5:1;
[0028] 4. After the reaction system is cooled to below 50°C, add 1460g of pure water and continue to stir. After the temp...
Embodiment 2
[0035] The preparation of embodiment 2 triamterene intermediate
[0036] 1. Add 420.7g of pure water and 201.4g of propanedinitrile to the reaction kettle in sequence. After stirring for 35 minutes to dissolve, turn on nitrogen protection, add 517.6g of aqueous solution containing 221.6g of sodium nitrite at 15°C, and add 8% HCl dropwise to adjust pH3~4, after the sodium nitrite aqueous solution is added dropwise, dilute hydrochloric acid consumes 90.2g, and keep warm at 15~20°C for 2h;
[0037] 2. Add 307.1g guanidine hydrochloride solid to 1, add 32.7g NaCO3 solid to adjust pH=10;
[0038] 3. Add 2000ml of a mixed solvent of dimethyl sulfoxide and xylene, heat up to reflux with water, keep warm at 140°C for 3 hours, and prepare for filtration; the composition of the mixed solvent: dimethyl sulfoxide: xylene is 5:1;
[0039] 4. After the reaction system is cooled to room temperature, add 2000g of pure water, continue to stir, and filter; the filter cake is rinsed with deioni...
Embodiment 3
[0046] The preparation of embodiment 3 triamterene intermediate
[0047] 1. Add 597.8g of pure water and 303.9g of propanedinitrile to the reaction kettle in turn. After stirring for 34min to dissolve, turn on the nitrogen protection, add 773.9 aqueous solution containing 335.1g of sodium nitrite at 15°C, and add 8% HCl dropwise to adjust the pH3 ~4. After the sodium nitrite aqueous solution is added dropwise, 147.6g of dilute hydrochloric acid is consumed, and kept at 18°C for 2.5h;
[0048] 2. Add 464.7g guanidine hydrochloride solid to 1, stir for 30min; add 50.7g NaCO3 solid to adjust pH=10;
[0049] 3. Add 2400ml of a mixed solvent of dimethyl sulfoxide and xylene, heat up to reflux with water, keep warm at 140°C for 4 hours, and prepare for filtration; the composition of the mixed solvent: dimethyl sulfoxide: xylene is 5:1;
[0050] 4. When the reaction system is cooled below 50°C, add 5000g of pure water and continue to stir. After the temperature cools down to 30°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com