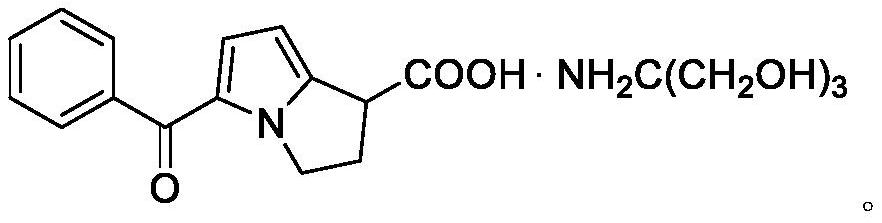
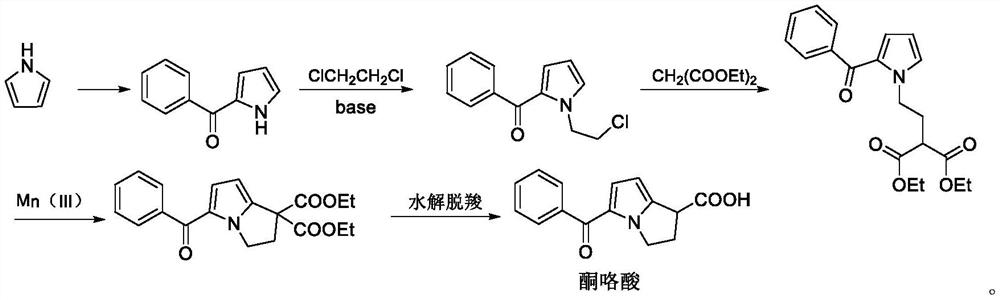
Preparation method of ketorolac tromethamine intermediate
A technology of ketorolac trometamol and intermediates, applied in organic chemistry and other fields, can solve the problems of being unsuitable for industrialized large-scale production, and achieve the effects of less impurities, improved reaction yield and purity, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
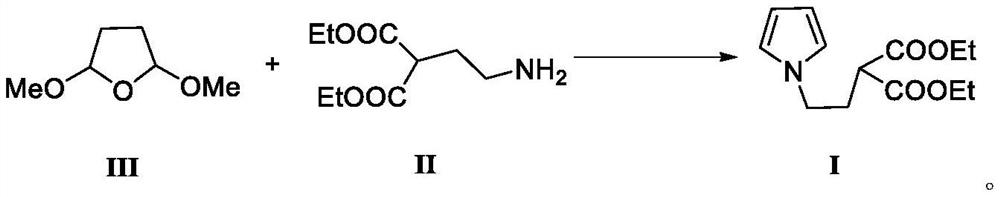
[0029] 2,5-dimethoxytetrahydrofuran (13.2g, 0.1mol), 2-(2-aminoethyl)-diethyl malonate (24.36g, 0.12mol), copper sulfate (1g, 8%) , tetrahydrofuran (100ml), and water (5ml) were added to a 250ml reaction flask, refluxed for 10h, cooled, evaporated to remove the solvent under reduced pressure, the residue was added dichloromethane (100ml), suction filtered, and the filtrate was washed with water (50ml). Separate layers, add anhydrous sodium sulfate to the dichloromethane layer for drying, and evaporate the solvent to give 2-(2-(1H-pyrrol-1-yl)ethyl)diethyl malonate with a yield of 98.7%, HPLC purity 99.88%.
Embodiment 2
[0031] 2,5-dimethoxytetrahydrofuran (13.2g, 0.1mol), 2-(2-aminoethyl)-diethyl malonate (22.33g, 0.11mol), copper acetate (1g, 8%) , acetonitrile (100ml), and water (5ml) were added to a 250ml reaction flask, refluxed for 10h, cooled, and the solvent was evaporated under reduced pressure, the residue was added chloroform (100ml), suction filtered, and the filtrate was washed with water (50ml). Separate layers, add anhydrous sodium sulfate to the chloroform layer to dry, and evaporate the solvent to give 2-(2-(1H-pyrrol-1-yl)ethyl)diethyl malonate with a yield of 94.4%, HPLC purity 99.82%.
Embodiment 3
[0033] 2,5-dimethoxytetrahydrofuran (13.2g, 0.1mol), 2-(2-aminoethyl)-diethyl malonate (20.32g, 0.10mol), copper chloride (1g, 8% ), tetrahydrofuran (100ml), water (5ml) were added to a 250ml reaction flask, refluxed for 10h, lowered the temperature, evaporated the solvent under reduced pressure, added chloroform (100ml) to the residue, suction filtered, and washed the filtrate with water (50ml) , layered, the chloroform layer was dried by adding anhydrous sodium sulfate, and the solvent was evaporated to dryness to obtain 2-(2-(1H-pyrrol-1-yl)ethyl)diethyl malonate with a yield of 86.8%, HPLC 99.74% pure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com