New process for synthesizing racemic naproxen based on Heck coupling
A new process and process technology, applied in the new process field based on Heck coupling synthesis of racemic naproxen, can solve the problems of difficult preparation of 2-acyl-6-methoxynaphthalene, interference product purification, low conversion rate, etc. , to achieve the effect of easy product purification, low cost and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of new technology based on Heck coupling synthesis racemic naproxen, the concrete steps of this technology are as follows:
[0033] (1) Synthesis of intermediate 3-(6-methoxynaphthyl-2-)crotonamide
[0034]
[0035] 24 grams (0.1mol) of 2-bromo-6-methoxynaphthalene, 88 milligrams (0.5 mmol) of palladium chloride, 304 milligrams (1 mmol) of three (2-methylphenyl)-phosphine, 13 grams (0.15 mol) of crotonamide and 20 g (0.2 mol) of triethylamine were dissolved in 100 mL of dimethylformamide, purged with nitrogen three times, then sealed and heated to 130°C, and kept stirring for 20 h.
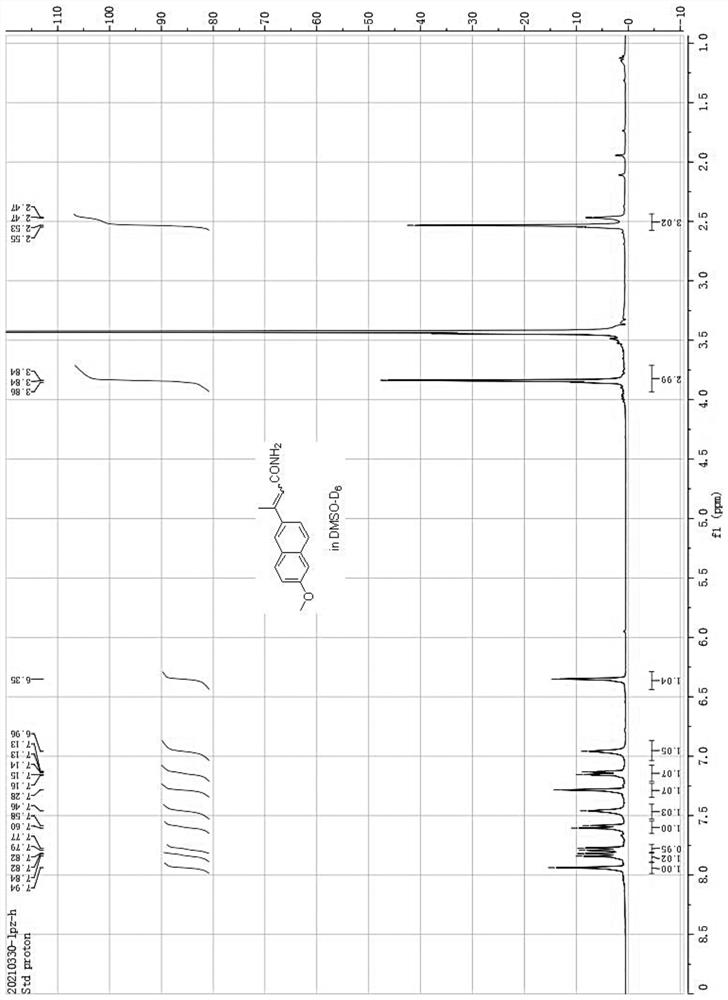
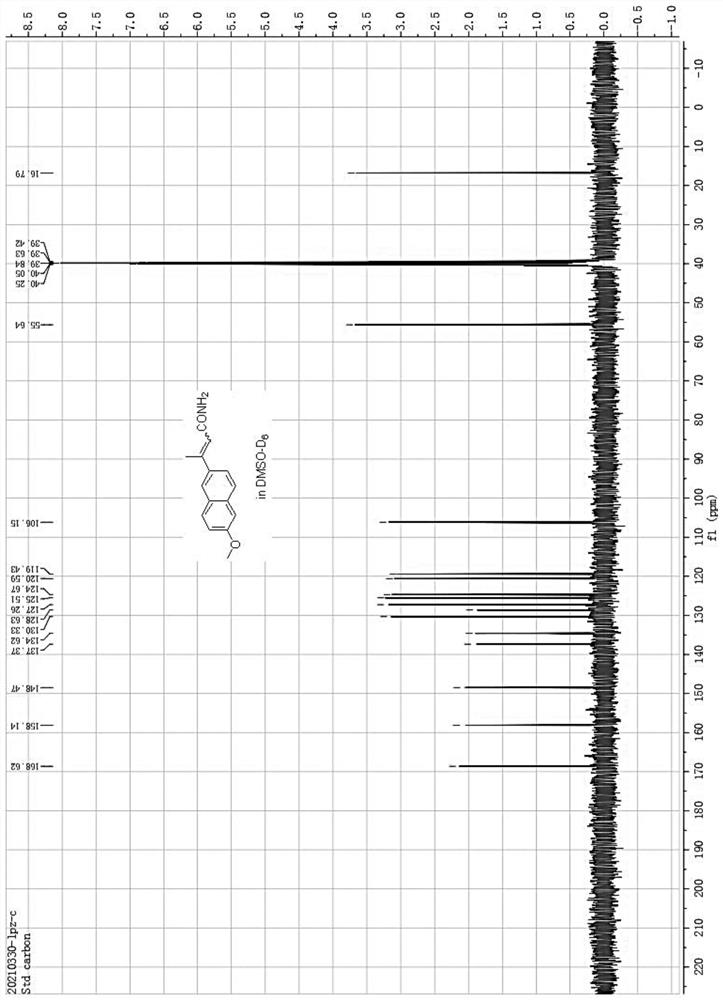
[0036] After the reaction is finished, cool down, filter the insoluble matter with diatomaceous earth, remove the solvent from the filtrate in vacuo, extract the residue twice with hot water, discard the water phase, and recrystallize the residue with ethanol to obtain 20.5 grams of 3-(6 -Methoxynaphthyl-2-)crotonamide (see H NMR spectrum and carbon spectrum figure 1 with 2 ), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com