Catalyst for methane dry gas reforming reaction and preparation method thereof
A dry gas reforming and catalyst technology, which can be used in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., and can solve the problems of increasing the thermal conductivity of oxide carriers, easy collapse of packaging materials, and limited stabilization. , to achieve the effects of improving heat transfer efficiency, improving carbon deposition resistance, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Dissolve 0.05g boric acid in 3ml water, then add 1g MgAl 2 o 4 Spinel, mixed and stirred until it became a paste and then dried for 12 hours to obtain MgAl modified with boron species 2 o 4 Prebody;
[0038] (2) the boron-containing species modified MgAl obtained in step (1) 2 o 4 Precursor, in NH 3 Under the atmosphere, the flow rate is 100ml / min, the temperature is raised to 800°C at 8°C / min and kept for 1h, and the temperature is raised to 1000°C at 2°C / min and kept for 1h to obtain MgAl 2 o 4 @h-BN;
[0039] (3) Dissolve 0.127g of nickel acetate in 2.5ml of water, add the MgAl obtained in step (2) 2 o 4 In @h-BN, stir and impregnate to a paste and then dry for 12 hours to obtain a catalyst precursor;
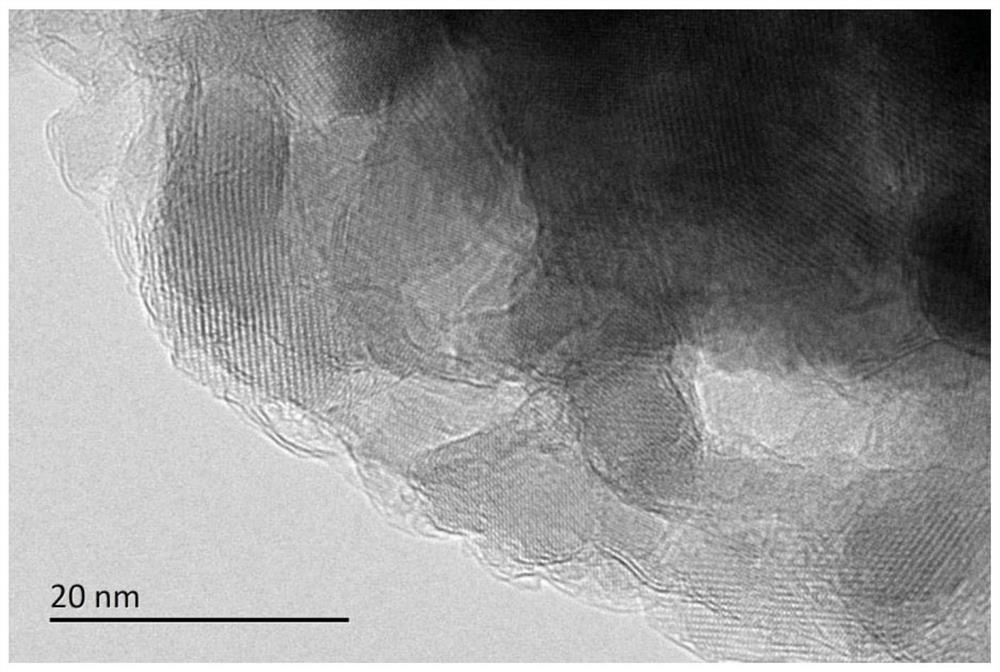
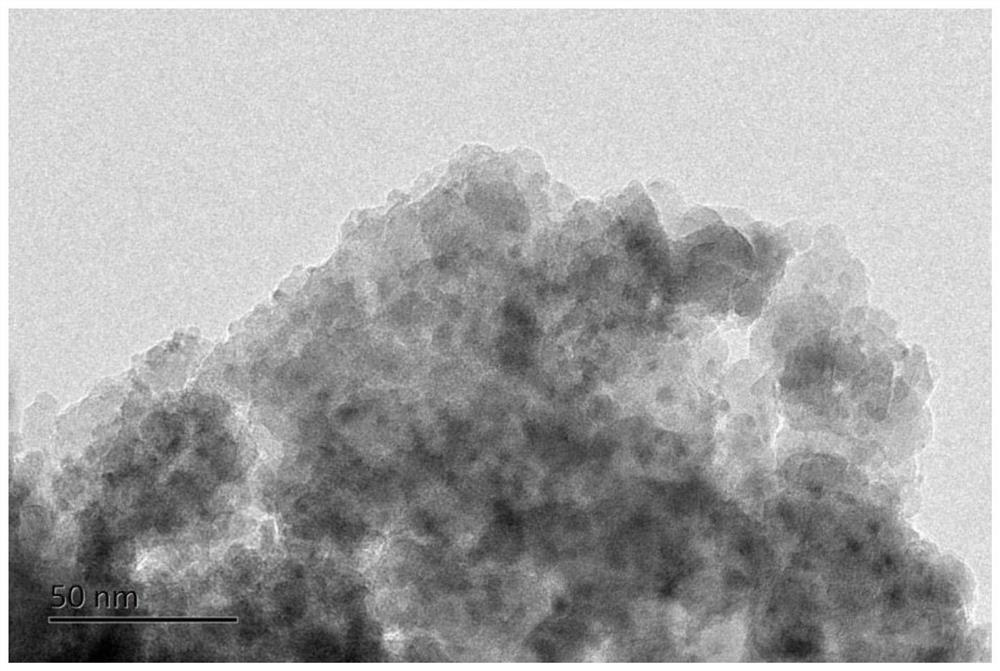
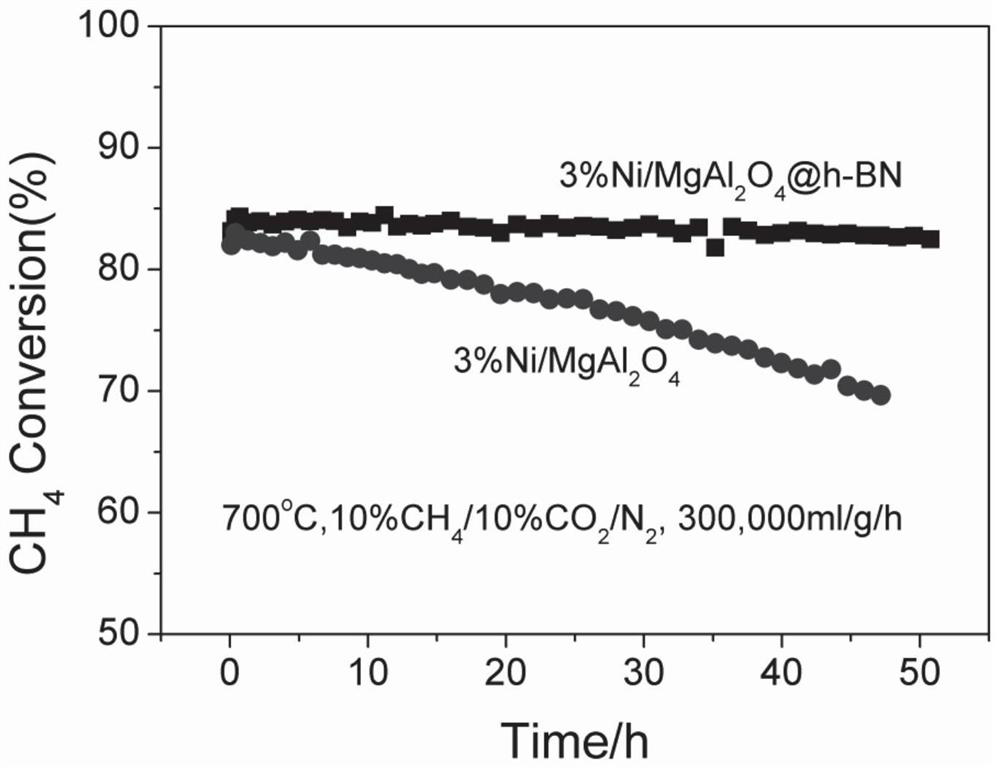
[0040] (4) Preform obtained in step (3) in H 2 Under atmosphere, heat up to 800°C at 1°C / min for 1h, H 2 Flow rate is 100ml / min, then lowers the temperature, obtains described methane dry reforming catalyst 3%Ni / MgAl 2 o 4 @h-BN.
[0041] For the ca...
Embodiment 2
[0047] (1) Dissolve 0.05g boric acid in 3ml water, then add 1g MgAl 2 o 4 Spinel, mixed and stirred until it became a paste and then dried for 12 hours to obtain MgAl modified with boron species 2 o 4 Prebody;
[0048] (2) the boron-containing species modified MgAl obtained in step (1) 2 o 4 Precursor, in NH 3 Under the atmosphere, the flow rate is 100ml / min, the temperature is raised to 800°C at 8°C / min and kept for 1h, and the temperature is raised to 1000°C at 2°C / min and kept for 1h to obtain MgAl 2 o 4 @h-BN;
[0049] (3) 0.424g of nickel acetate is dissolved in 3ml of water, and the MgAl obtained in step (2) is added 2 o 4 In @h-BN, stir and impregnate to a paste and then dry for 12 hours to obtain a catalyst precursor;
[0050] (4) Preform obtained in step (3) in H 2 Under atmosphere, heat up to 800°C at 1°C / min for 1h, H 2 Flow rate is 100ml / min, then lowers the temperature, obtains described methane dry reforming catalyst 10%Ni / MgAl 2 o 4 @h-BN. The res...
Embodiment 3
[0052] (1) Dissolve 0.2g boric acid in 3ml water, then add 1g SiO 2 , mixed and stirred to paste and then dried for 12h to obtain SiO modified with boron species 2 Prebody;
[0053] (2) SiO modified with boron-containing species obtained in step (1) 2 Precursor, in NH 3 Under the atmosphere, the flow rate is 100ml / min, the temperature is raised to 800°C at 8°C / min and kept for 1h, and the temperature is raised to 1000°C at 2°C / min and kept for 1h to obtain SiO 2 @h-BN;
[0054] (3) Dissolve 0.424g nickel acetate in 3ml water, add SiO obtained in step (2) 2 In @h-BN, stir and impregnate to a paste and then dry for 12 hours to obtain a catalyst precursor;
[0055] (4) Preform obtained in step (3) in H 2 Under atmosphere, heat up to 800°C at 1°C / min for 1h, H 2 Flow rate is 100ml / min, then lowers the temperature, obtains described methane dry reforming catalyst 10%Ni / SiO 2 @h-BN. The resulting Ni particle size is around 2nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com