Anti-ST2 antibody and use thereof
An antibody and L-CDR2 technology, applied in the direction of antibodies, applications, anti-inflammatory agents, etc., can solve the problems of differences in clinical efficacy, dosage, and biological activity of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0215] Example 1: Murine Antibody Screening
[0216] 1.1 Animal immunity
[0217] CHO-K1 cells were used to express Human ST2-his, and then 6 BALB / c mice were immunized according to the routine Freund's adjuvant immunization procedure. The immunization was carried out in two batches, 3 mice in each batch, with an interval of two weeks between the two batches. Each batch of mice was immunized 4 times, and the Human ST2-his was used for ELISA detection. If the mouse serum titer was >1:100,000, the final immunization was performed, and the spleen was collected 3-4 days later.
[0218] 1.2B cell panning and culture
[0219] 2 days before the formal experiment, the feeder cells were spread into four 10cm culture dishes (Corning, cat. 430167), and the feeder cells were treated with 25 μg / mL MMC for 6 hours 1 day before the formal experiment, and then 10000 cells / The wells of a 96-well plate (Corning, cat.3599) were paved, 100 μL / well; at the same time, a 6-well plate was used ...
Embodiment 2
[0229] Example 2: Antibody engineering
[0230] 2.1 B cell sequencing
[0231] Follow PureLink TM The RNA Mini Kit manual extracts the mRNA of B cell clones, and stores in -80°C in aliquots; the extracted mRNA is used as a template using PrimeScript TM II 1st Strand cDNA Synthesis Kit protocol reverse transcribed into cDNA, and stored in -80 ℃.
[0232] Use the heavy chain VH fishing primers and light chain VL fishing primers shown in Table 1-1 and Table 1-2, use the above cDNA as a template, use Ex Taq enzyme to amplify VH and VL, and then connect to pMD18T vector for sequencing .
[0233] Table 1-1 Heavy chain VH fishing primers
[0234]
[0235]
[0236] Table 1-2 Light chain VL fishing primers
[0237]
[0238]
[0239] 2.2 Recombinant expression and screening of mouse antibody
[0240] (1) Mouse anti-recombinant expression
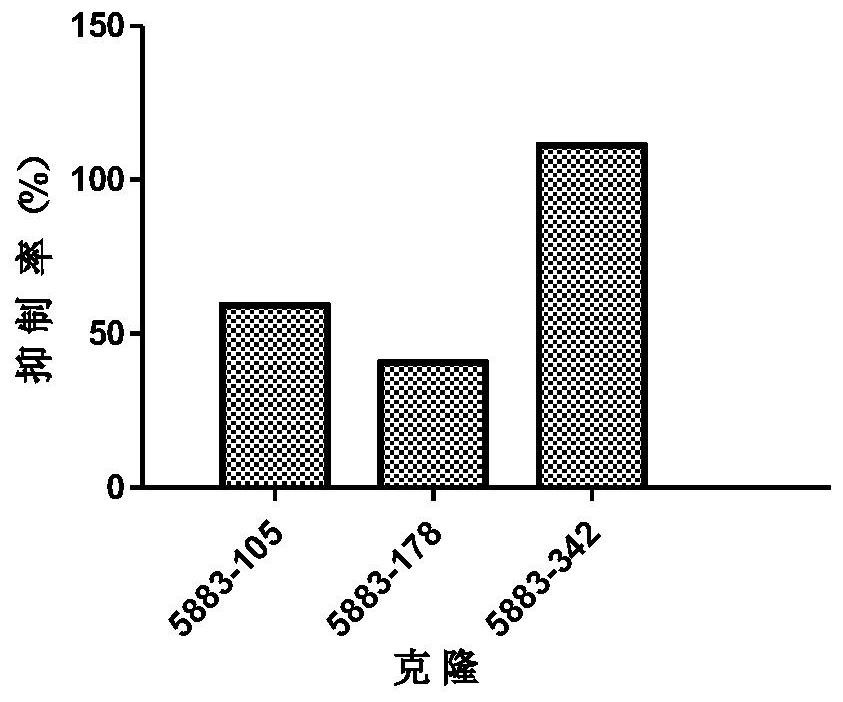
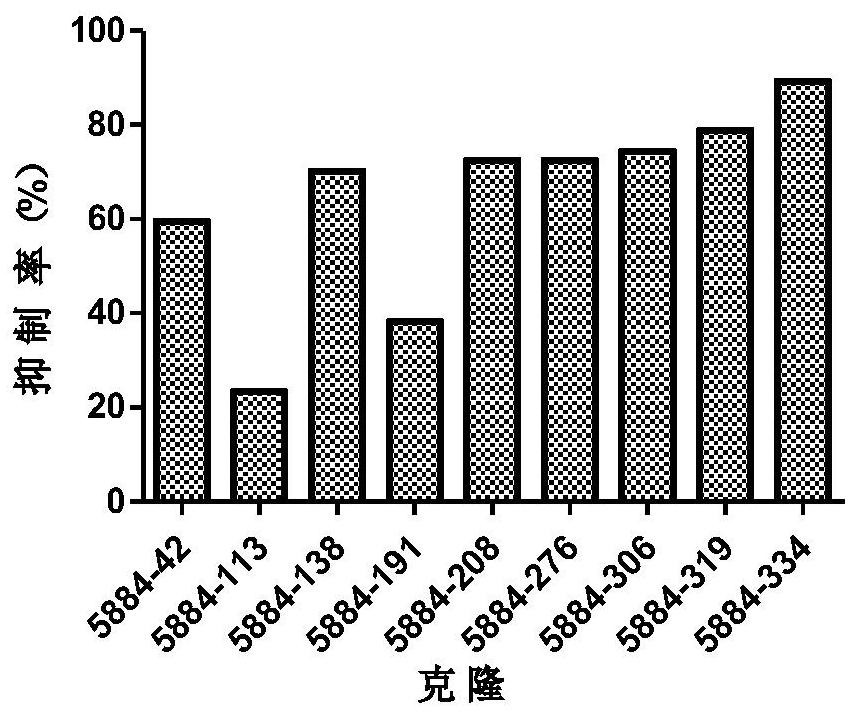
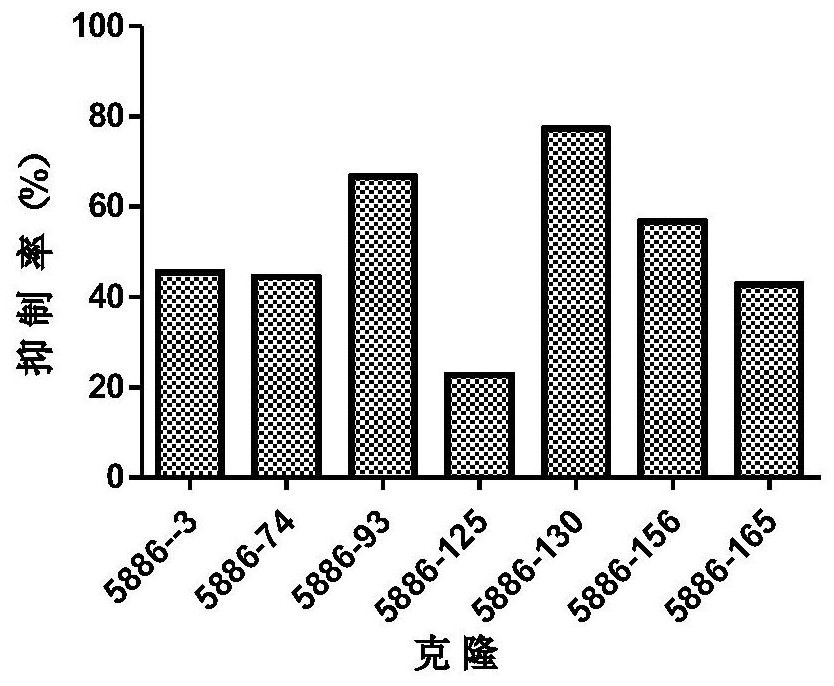
[0241] will come from the same clone (eg Figure 1 to Figure 5 The light and heavy chains shown in ) were combined to transfect ...
Embodiment 3
[0369] Example 3 Antibody Mouse PK
[0370] After the adaptation period, 20 mice were randomly divided into 4 groups, 5 in each group, and the administration information is shown in Table 31.
[0371] Table 31 Administration information of mice in each group
[0372] Antibody Method of administration Dosing volume A 5888-116-H0L1 5mg / kg i.p. 0.1mL / 10g B 5888-153-H0L1 5mg / kg i.p. 0.1mL / 10g C CNTO7160 5mg / kg i.p. 0.1mL / 10g D 5886-156-H1L0 5mg / kg i.p. 0.1mL / 10g
[0373] Drugs were administered according to the groups, and the time of administration was recorded. Each group of mice before administration (0hr) and after administration 4hr, 8hr, 24hr(1d), 72hr(3d), 120hr(5d), 168hr(7d), 240hr(10d), 288hr(12d), 336hr (14d) Blood was collected separately, and serum was collected and stored at -60 to -80°C.
[0374]The detection method of PK blood drug concentration in mice is as follows:
[0375] (1) Coating: Human ST2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com