Novel laurolactam preparation method and synthesis apparatus
A technology of laurolactam and synthesis equipment, which is applied in the preparation of lactam, oxime and heterocyclic compounds, etc., to achieve the effects of high purity, high conversion rate, high selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
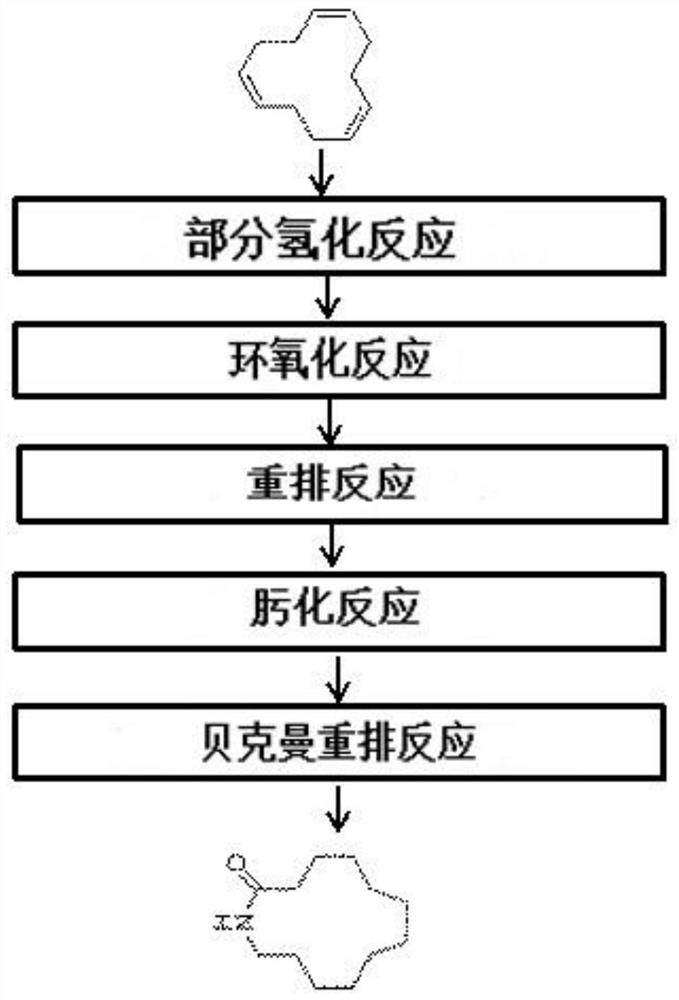
[0035] The present invention provides a method for preparing laurolactam, a synthesis device thereof, and a system for preparing laurolactam including one or more of them. When performing each process step of the present invention, the following effects can be achieved, that is, There is no need to separate the obtained product including the target substance synthesized in each step without reacting and remaining substances, etc. in a separate process, and the above obtained product can be directly used as a reactant in the subsequent step, and the conversion rate and selectivity can reach significantly higher until the final step of the synthesis of laurolactam.
[0036] The preparation method of laurolactam of the present invention comprises: step a), utilizes catalyst to epoxidize cyclododecene to synthesize epoxidized cyclododecane; Step b), by making above-mentioned epoxidized cyclododecane performing a catalytic reaction to synthesize cyclododecanone; and step c), synthe...
Embodiment 1
[0080] Cyclododecene Synthesis Process
[0081] Utilize high-speed stirring batch type (Batch) reactor (500ml, 800rpm), the cyclododecatriene of 200g, the RuCl of 40mg 3 , 5.56g of triphenylphosphine (110:1=triphenylphosphine:Ru), 3.44g of 35% formalin (triphenylphosphine:formalin=1:2), 0.5g of acetic acid (Acetic acid), 10.54 g of ethanol were added to the reactor, and the reactor was connected. After that, use 5kg / cm 2 Nitrogen (N 2 ) purging (purge) 3 times, using hydrogen (H 2 ) after purging 3 times, the reactor pressure was increased to 10 barg. Next, increase the reactor temperature from 25°C to 145°C in about 40 minutes, if the pressure of the reactor starts to drop, raise the reactor pressure to 20bar, raise the temperature to 160°C in about 10 minutes, and carry out the reaction Keep it in the process. The reaction was carried out for a total of 6 hours, during which time hydrogen was continuously supplied in order to continue to maintain the pressure. After...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com