Plasma preserving fluid and application thereof
A technology of preservation solution and plasma, which can be used in applications, preservation of human or animal bodies, animal husbandry, etc., and can solve problems such as melting, changes in component properties, and absence of plasma quality control products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of plasma preservation solution
[0023] Prepare 5 groups of plasma preservation solutions with water for injection according to the following ingredients.
[0024] (1) Experimental group 1: glucose 5g / L, citric acid 0.55g / L, sodium citrate 8g / L, sodium chloride 1.6g / L, disodium hydrogen phosphate 0.56g / L, sodium dihydrogen phosphate 0.5 g / L, bovine serum albumin 10g / L, mannitol 10g / L, trehalose 10g / L, sodium azide 1g / L and β-cyclodextrin 1mg / L.
[0025] (2) Experimental group 2: Change the concentration of β-cyclodextrin to 10mg / L, and the concentration of other components in experimental group 1.
[0026] (3) Experimental group 3: Change the concentration of β-cyclodextrin to 50mg / L, and the concentration of other components in experimental group 1.
[0027] (4) Experimental group 4: Change the concentration of β-cyclodextrin to 100mg / L, and the concentration of other components in experimental group 1.
[0028] (5) Experimental group 5: Chang...
Embodiment 2
[0029] The preparation of embodiment 2 ABO blood group antitype plasma quality control product
[0030] Aspirate 9 mL of the plasma (that is, the supernatant after centrifugation) that has been processed by the aforementioned plasma processing method and add it to blood collection tubes containing 1 mL of the plasma preservation solution prepared in Example 1 without anticoagulants to obtain various plasma preservation solutions. Prepared ABO blood group reverse type plasma quality control. For each plasma preservation solution, prepare two tubes of ABO blood type reverse plasma quality control products, hereinafter referred to as "quality control products", one of which is used for testing, and the other tube is used for static observation. Store in refrigerator at 2-8°C.
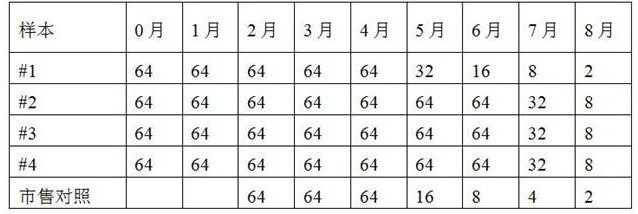
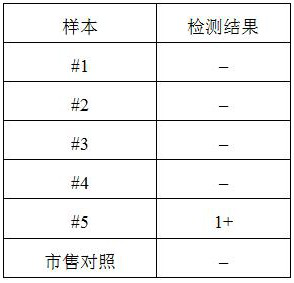
[0031] For the convenience of illustration, the plasma quality control products prepared in the preservation solution of Experimental Group 1-5 in Example 1 are correspondingly marked as "#1" to "#5" here...
Embodiment 3
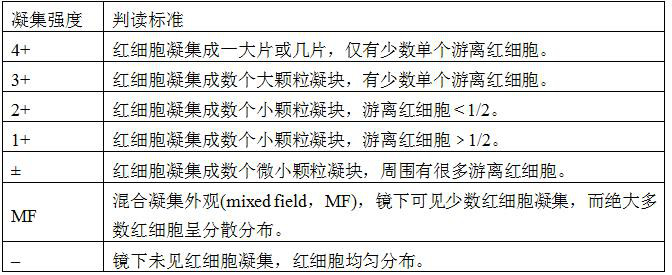
[0032] Example 3 Specificity and antibody titer detection of each plasma quality control product
[0033] The test tube method was used to detect the specificity and antibody titer of each plasma quality control product, and the reference was the "Clinical Blood Transfusion Test Operation Procedures". The specificity and antibody titer of the A-type quality controls stored in each preservation solution were determined, and compared with the A-type serum quality controls in the commercially available ABO and RhD blood group detection quality controls. The commercially available ABO and RhD blood type test quality control products were purchased from Changchun Boxun Biotechnology Co., Ltd., the specification is: 6 bottles / box (article number: BX7001), and the label indicates 2-8°C storage, and the validity period is 90 days .
[0034] The specific detection method is briefly described as follows. Take 6 test tubes and mark them as 1, 2, 3, 4, 5 and the commercially available c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com