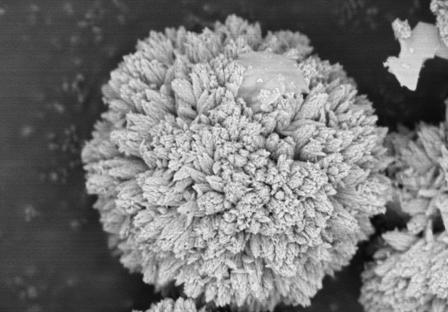
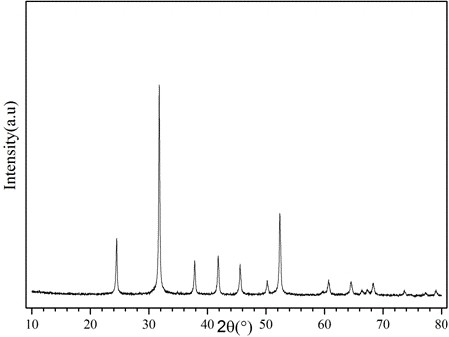
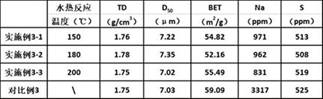
A kind of carbonate precursor and preparation method thereof
A carbonate, precursor technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] ① Preparation of feed solution: Dissolve manganese sulfate, cobalt sulfate, nickel sulfate, and magnesium sulfate in deionized water according to the stoichiometric molar ratio Mn:Co:Ni:A=0.70:0.15:0.15:0.01 to prepare the total metal ion concentration It is a 2mol / L mixed metal ion solution. Sodium carbonate was dissolved in deionized water to prepare a 2mol / L precipitant solution. Sodium fluoride was dissolved in deionized water to prepare a 0.2mol / L complexing agent solution.
[0041] ② Co-precipitation reaction: In the nucleation reaction stage, the sodium carbonate solution and the mixed metal ion solution are adjusted according to the molar ratio of the carbonate ion to the total metal ion = 3:1 to adjust the flow rate of the pumped sodium carbonate solution and the mixed metal ion solution, and complex The concentration of the agent in the total feed solution is controlled at 0.01mol / L, and pumped into a 100L continuous stirring reactor at the same time, which c...
Embodiment 2
[0052] ① Preparation of feed solution: Manganese sulfate, cobalt sulfate, nickel sulfate, and aluminum sulfate are dissolved in deionized water according to the stoichiometric ratio of Mn: Co: Ni: A=0.60:0.20:0.20:0.01 to prepare a total metal ion concentration of 2mol / L mixed metal ion solution. Sodium carbonate was dissolved in deionized water to prepare a 2mol / L precipitant solution. Sodium fluoride was dissolved in deionized water to prepare a 0.2mol / L complexing agent solution.
[0053] ② Co-precipitation reaction: In the nucleation reaction stage, the sodium carbonate solution and the mixed metal ion solution are adjusted according to the molar ratio of carbonate ions and total metal ions to 5:1, and the pumping flow rate of the materials is adjusted. The complexing agent is in the total feed solution The concentration is controlled at 0.012mol / L, and pumped into a 100L continuous stirring reactor at the same time, which contains 50L sodium carbonate solution as the bot...
Embodiment 3
[0066] ① Preparation of feed solution: Manganese sulfate, cobalt sulfate, nickel sulfate, magnesium sulfate are dissolved in deionized water according to the stoichiometric ratio Mn: Co: Ni: A=0.50:0.20:0.30:0.01 to prepare a total metal ion concentration of 2mol / L mixed metal ion solution. Sodium carbonate was dissolved in deionized water to prepare a 2mol / L precipitant solution. Sodium fluoride was dissolved in deionized water to prepare a 0.2mol / L complexing agent solution.
[0067] ② Co-precipitation reaction: In the nucleation reaction stage, the sodium carbonate solution and the mixed metal ion solution are adjusted according to the molar ratio of carbonate ions and total metal ions to 3:1, and the pumping flow rate of the materials is adjusted. The complexing agent is in the total feed solution The concentration of the solution is controlled at 0.01mol / L, and it is pumped into a 100L continuous stirring reactor, which contains 50L sodium carbonate solution as the botto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com