Method for preparing ketone compound from olefin
A technology of ketone compounds and olefins, applied in the field of organic chemical synthesis, can solve problems such as high cost, environmental problems, and limitations on the practicality of Wacker reactions, and achieve the effect of simple synthesis and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
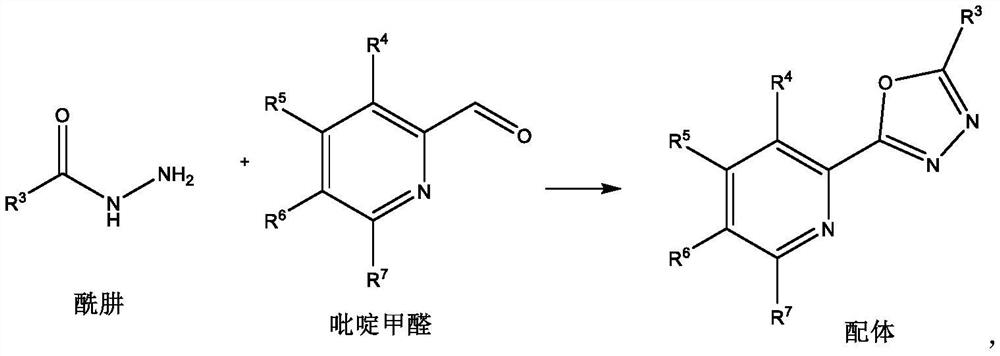
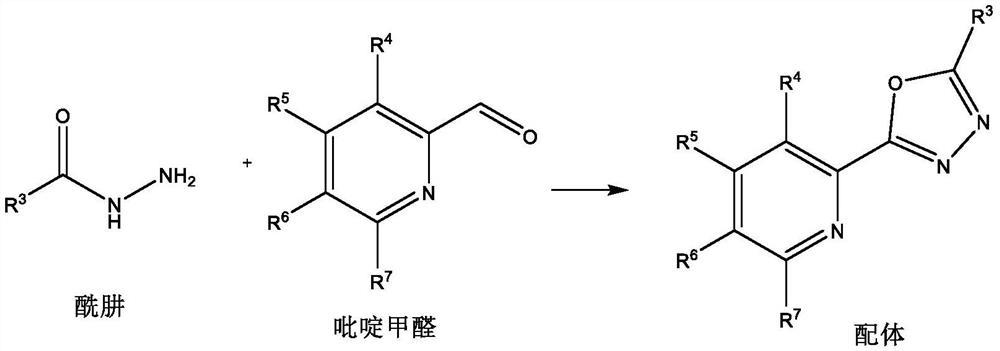
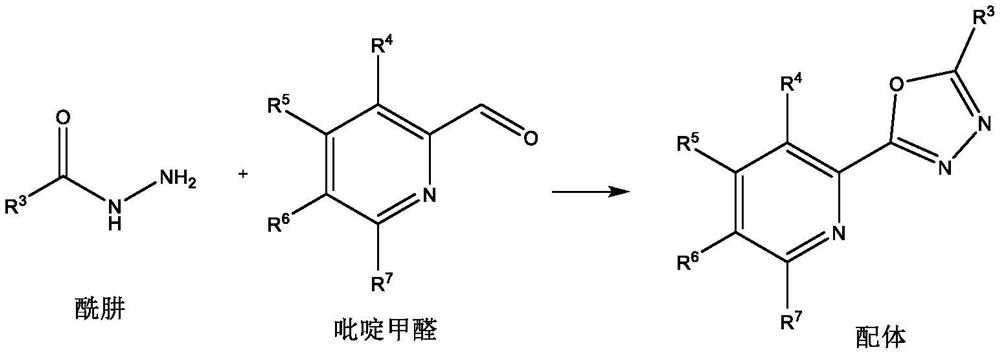
[0015] Ligand preparation method:
[0016]
[0017] Add hydrazide (10mmol), pyridine formaldehyde (10mmol) and solvent methanol (20mL) into the reaction flask, add elemental iodine (0.5mmol) under stirring, and reflux reaction in the presence of air for 8 hours. After the reaction, add water and ethyl acetate The ester was extracted three times. The water layer was removed, and the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and recrystallized with 20 mL of ethanol to obtain the ligand.
Embodiment 1
[0018] Embodiment 1 (preparation of ligand L1)
[0019]
[0020] The hydrazide is selected from acetylhydrazide, the pyridine formaldehyde is selected from pyridine-2-carbaldehyde, and the ligand L1 is prepared according to the method of implementation one. 1 HNMR: δ8.59(d, J=7.5Hz, 1H), 8.01(d, J=7.5Hz, 1H), 7.85(dd, J=7.5, 7.5Hz, 1H), 7.42(dd, J=7.5, 7.5Hz,1H),2.63(s,3H). 13 C NMR: δ164.7, 164.5, 157.4, 149.2, 137.2, 124.2, 123.6, 20.5.
Embodiment 2
[0021] Embodiment 2 (preparation of ligand L2)
[0022]
[0023] The hydrazide is selected from benzohydrazide, the pyridine formaldehyde is selected from 4-methoxypyridine-2-carbaldehyde, and the ligand L2 is prepared according to the method of implementation one. 1 H NMR: δ8.61(d, J=7.5Hz, 1H), 7.98(m, 2H), 7.73(d, J=7.5Hz, 1H), 7.62(m, 3H), 7.43(s, 1H), 3.81(s,3H). 13 C NMR: δ164.8, 164.4, 159.4, 158.3, 150.2, 133.7, 129.3, 129.1, 128.7, 127.7, 127.5, 107.9, 105.4, 55.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com