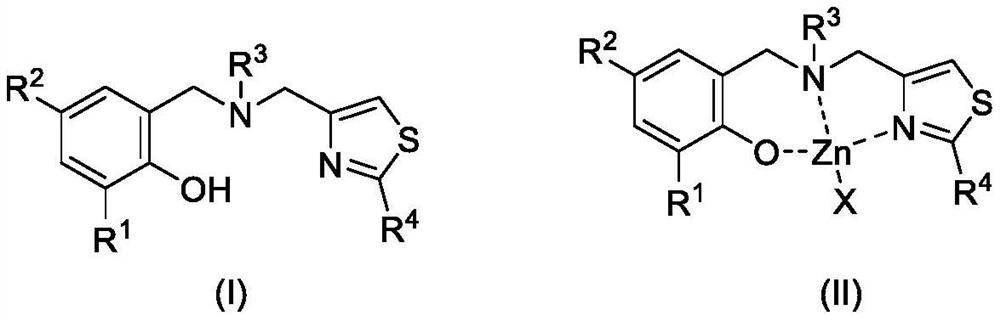
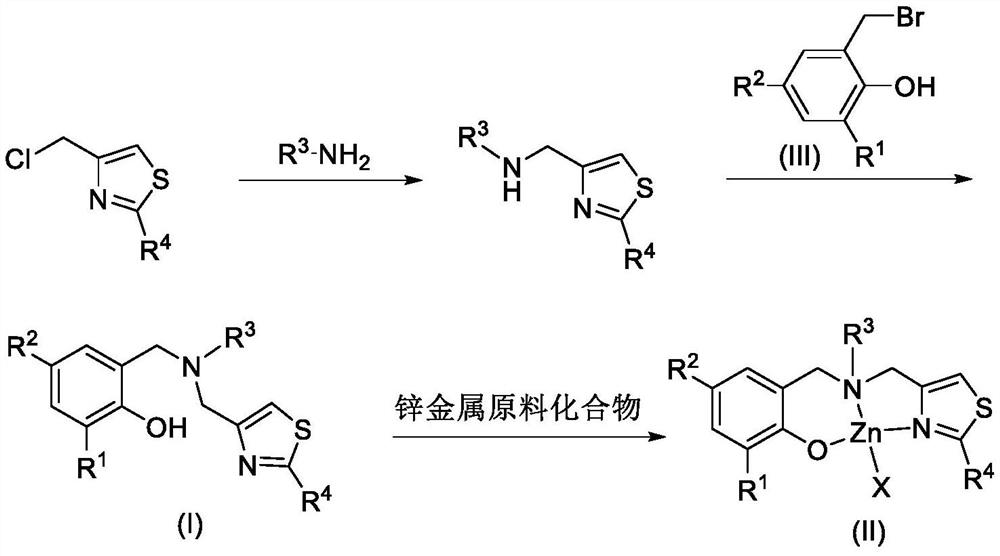
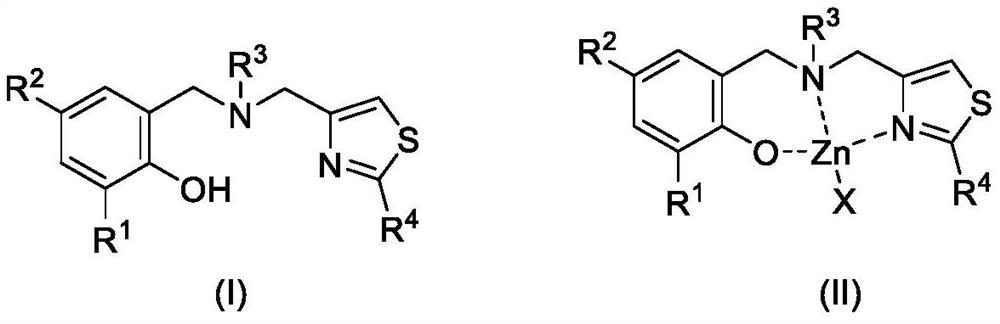
2-substituted thiazole-4-yl-containing aminophenol oxygroup zinc complex as well as preparation method and application of 2-substituted thiazole-4-yl-containing aminophenol oxygroup zinc complex
A technology of aminophenoloxyzinc and aminophenol, which is applied in the field of aminophenoloxyzinc complexes, can solve the problems of low catalytic activity, no stereoselectivity, and needs to be further deepened
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Ligand L 1 Synthesis of H:
[0046] (1) Synthesis of N-[(2-phenylthiazol-4-yl)methyl]n-hexylamine
[0047]
[0048] Dissolve 2-phenyl-4-chloromethylthiazole (10.7mmol, 2.24g) in 30mL dry DMF, add n-hexylamine (107mmol, 14mL), and finally add anhydrous K 2 CO 3 (11.8mmol, 1.63g), the reaction was stirred overnight. Quench the reaction with water, extract with ethyl acetate, wash the organic phase with saturated brine, then dry with anhydrous sodium sulfate, spin off the solvent to obtain a yellow oily mixture, and remove excess n-hexylamine under reduced pressure at 110°C / 8mmHg to obtain brown Crude product of red transparent oily liquid.
[0049] The purity is greater than 95%, and the yield is about 80%
[0050] (2) Ligand L 1 Synthesis of H
[0051] In a 100mL round bottom flask, add the above secondary amine (2.35g, about 8.56mmol), anhydrous K 2 CO 3 (1.3g, 9.4mmol), dissolved in 30mL DMF, added 2-bromomethyl-4-methyl-6-tritylphenol (4.2g, 9.0mmol), and ...
Embodiment 2
[0055] Ligand L 2 Synthesis of H
[0056] (1) Synthesis of N-[(2-phenylthiazol-4-yl)methyl]benzylamine
[0057]
[0058] Except that benzylamine (21.8mL, 200mmol), potassium carbonate (3.0g, 22mmol) and 2-phenyl-4-chloromethylthiazole (4.2g, 20mmol) were used as raw materials, other operating steps were the same as in Example 1. Excess benzylamine was removed under reduced pressure and heating (100° C. / 8 mmHg) to obtain 4.5 g of a brownish-red transparent liquid as a crude product with a purity greater than 95% and a yield of about 80%.
[0059] (2) Ligand L 2 Synthesis of H
[0060]N-[(2-phenylthiazol-4-yl)methyl]benzylamine (4.48g, about 16mmol), anhydrous potassium carbonate (2.43g, 17.6mmol) and 2-bromomethyl-4- Except for methyl-6-tritylphenol (7.54g, 17mmol), other operations were the same as in Example 1, and recrystallized from dichloromethane and methanol to obtain a white solid powder (5.9g, 57%).
[0061]
[0062] 1 H NMR (400MHz, CDCl 3 ):δ10.57(br s,1...
Embodiment 3
[0064] Ligand L 3 Synthesis of H
[0065] (1) Synthesis of N-[(2-tert-butylthiazol-4-yl)methyl]n-hexylamine
[0066]
[0067] Except that n-hexylamine (21.0 mL, 164 mmol), potassium carbonate (2.5 g, 18 mmol) and 2-tert-butyl-4-chloromethylthiazole (3.12 g, 16.4 mmol) were used as raw materials, other operating steps were the same as in Example 1. Excess n-hexylamine was removed under reduced pressure and heating (110° C. / 8 mmHg) to obtain 3.0 g of a brown-red transparent oil with a purity of more than 95% and a yield of about 71%.
[0068] (2) Ligand L 3 Synthesis of H
[0069] In addition to raw materials, N-[(2-tert-butylthiazol-4-yl)methyl]n-hexylamine (3.0g, about 11.7mmol), anhydrous potassium carbonate (1.8g, 12.9mmol) and 2-bromomethyl -Except for 4-methyl-6-tritylphenol (5.3g, 12.0mmol), other operations were the same as in Example 1, and recrystallized from petroleum ether and methanol to obtain a white solid (5.2g, 73%).
[0070]
[0071] 1 H NMR (400MHz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com