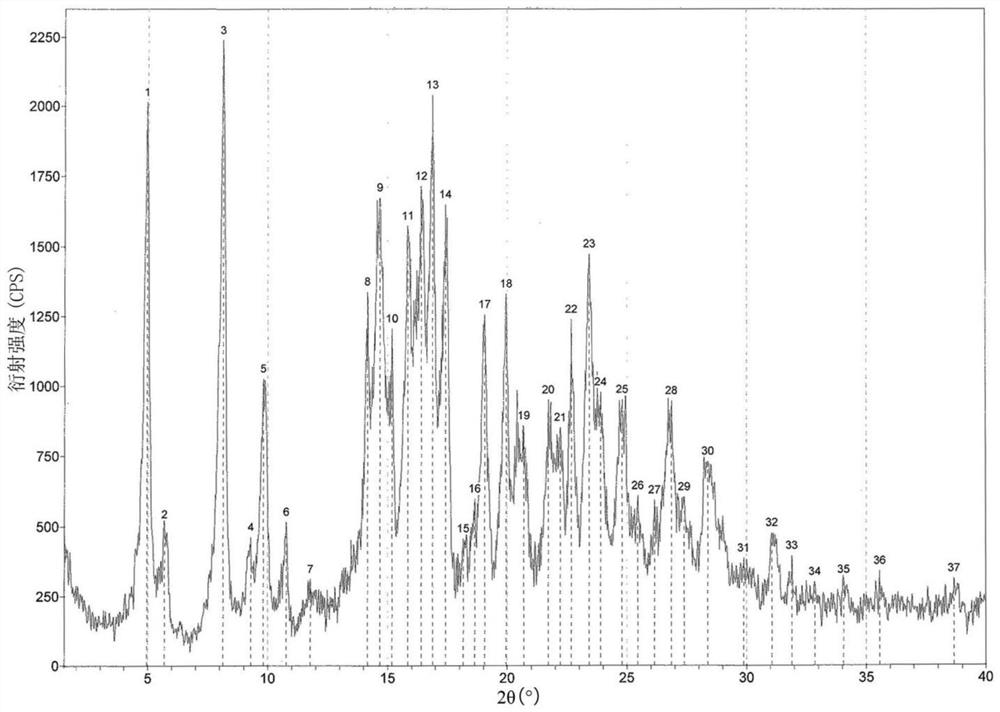
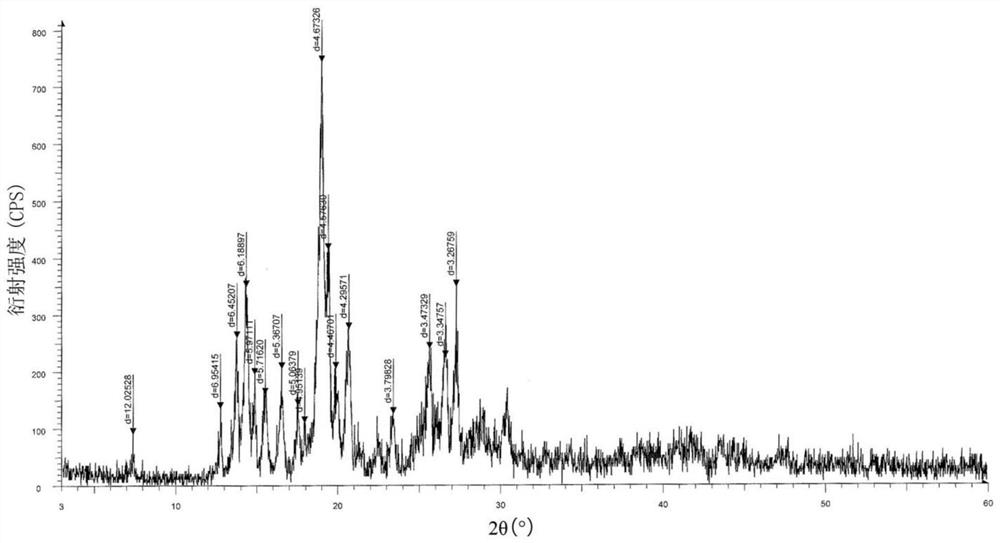
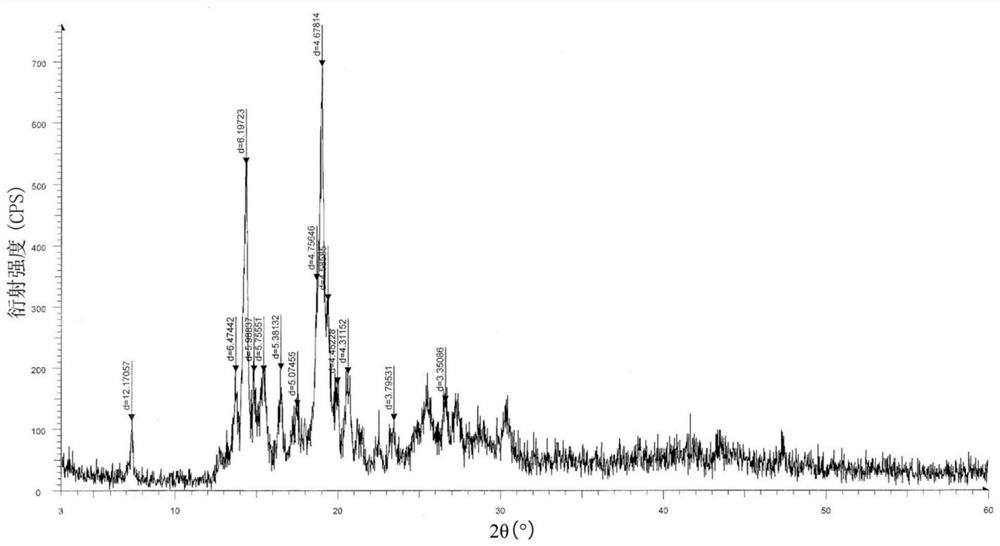
Recrystallization method of andrographolide C15-substituted derivative
A technology of andrographolide and derivatives, applied in the direction of organic chemistry and the like, can solve problems such as large amount of solvent, and achieve the effects of stable product quality, convenient operation and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example provides a recrystallization method of 15Z)15-(4-chlorophenyl)-methylene-11,12-dehydro-14-deoxyandrographolide.
[0034] Step a, add 14.85L (9vol) tetrahydrofuran to a 20L glass reactor, and add 1.65kg 15Z)15-(4-chlorophenyl)-methylene-11,12-dehydro-14-deoxyandrographis paniculata while stirring For the crude ester (content 80.3%), turn on the heating and heat up to reflux until the material is completely dissolved, add 132g of activated carbon, heat filter to remove carbon after reflux for 15min, transfer the filtrate to another 20L glass reactor, stir slowly and cool down to 0 Crystallization at ~5°C. Centrifuge and dry at 65° C. for 10 hours to obtain 866.2 g of the light yellow first solid, with a content of 99.91%, a purity of 99.73%, and a yield of 65.4%. The centrifuged mother liquor is transferred to a 100L glass-lined reaction tank for recovery. Wherein yield=first solid mass×first solid content / (crude product mass×crude product content)×100%.
...
Embodiment 2
[0038] This example provides a recrystallization method of 15Z)15-(4-chlorophenyl)-methylene-11,12-dehydro-14-deoxyandrographolide.
[0039] Step a, add 9.75L (6.5vol) tetrahydrofuran to a 20L glass reactor, and add 1.50kg15Z)15-(4-chlorophenyl)-methylene-11,12-dehydro-14-deoxyandrographis paniculata while stirring For the crude ester (content 78.9%), turn on the heating and heat up to reflux until the material is completely dissolved, add 90.0g of activated carbon, heat filter to remove carbon after reflux for 60min, transfer the filtrate to another 20L glass reactor, stir slowly and cool down to Crystallization at 0~5℃. Centrifuge and dry at 65° C. for 10 hours to obtain 840 g of the light yellow first solid, with a content of 99.97%, a purity of 99.68%, and a yield of 71.0%. The centrifuged mother liquor is transferred to a 100L glass-lined reaction tank for recovery. Wherein yield=first solid mass×first solid content / (crude product mass×crude product content)×100%.
[00...
Embodiment 3
[0043] This example provides a recrystallization method of 15Z)15-(4-chlorophenyl)-methylene-11,12-dehydro-14-deoxyandrographolide.
[0044] Step a, add 14.0L (8vol) THF to a 20L glass reactor, and add 1.75kg15Z)15-(4-chlorophenyl)-methylene-11,12-dehydro-14-deoxyandrographolide under stirring Crude product (content 77.2%), turn on heating and heat up to reflux until the material is completely dissolved, add 87.5g of activated carbon, heat filter to remove carbon after reflux for 45min, transfer the filtrate to another 20L glass reactor, stir slowly and cool down to 0 Crystallization at ~5°C. After centrifugation and air drying at 65° C. for 10 h, 920 g of the light yellow-green first solid was obtained, with a content of 99.94%, a purity of 99.71%, and a yield of 68.1%. ; The centrifuged mother liquor is transferred to a 100L glass-lined reaction tank to be recovered. Wherein yield=first solid mass×first solid content / (crude product mass×crude product content)×100%.
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com