Imidazotetrazine compounds
A compound and alkyl technology, applied in the field of imidazolium tetrazine compounds, can solve the problems of unclear maximum curative effect and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0155] The invention also relates to processes for the preparation of said compounds and compositions. The compounds and compositions can be prepared by any suitable technique of organic synthesis, such as those described herein. Many such techniques are known in the art. Many such known techniques are described in the following documents: Compendium of Organic Synthetic Methods (John Wiley & Sons, New York), Vol. 1, Ian T. Harrison and Shuyen Harrison, 1971; Vol. 2, Ian T. Harrison and Shuyen Harrison, 1974; Vol.3,Louis S.Hegedus and Leroy Wade,1977;Vol.4,Leroy G.Wade,Jr.,1980;Vol.5,Leroy G.Wade,Jr.,1984;and Vol.6,Michael B. Smith; and standard organic reference books such as March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 5 th Ed.by M.B.Smith and J.March (John Wiley&Sons, New York, 2001), Comprehensive Organic Synthesis; Selectivity, Strategy & Efficiency in Modern Organic Chemistry, in 9Volumes, Barry M.Trost, Ed.-in-Chief (Pergamon Press, New ...
Embodiment 1
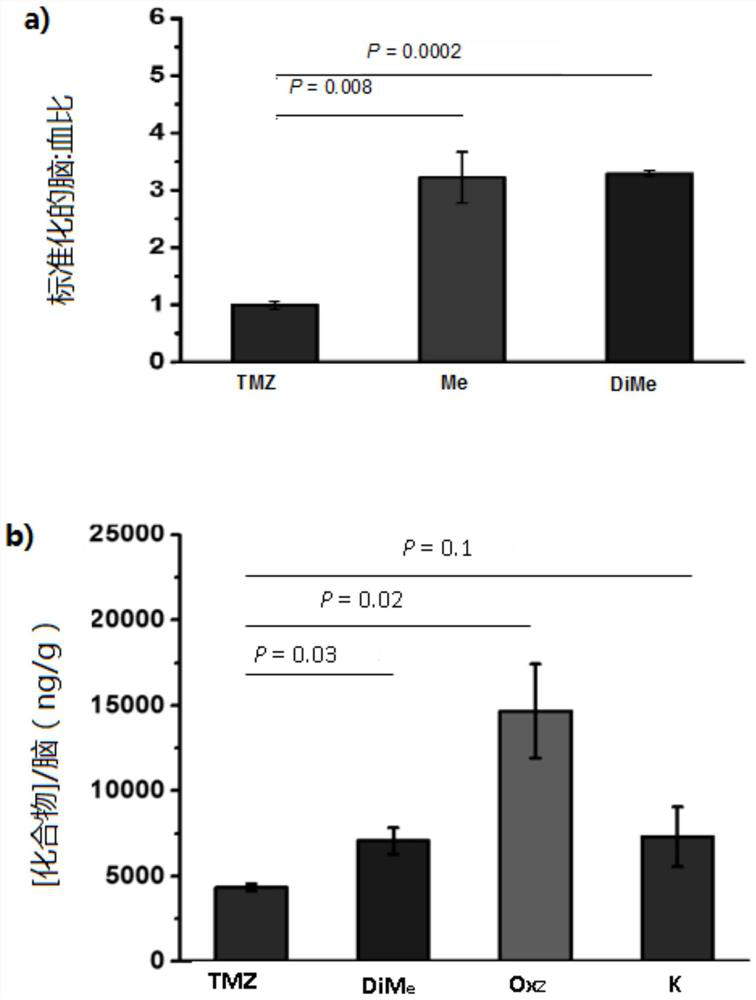
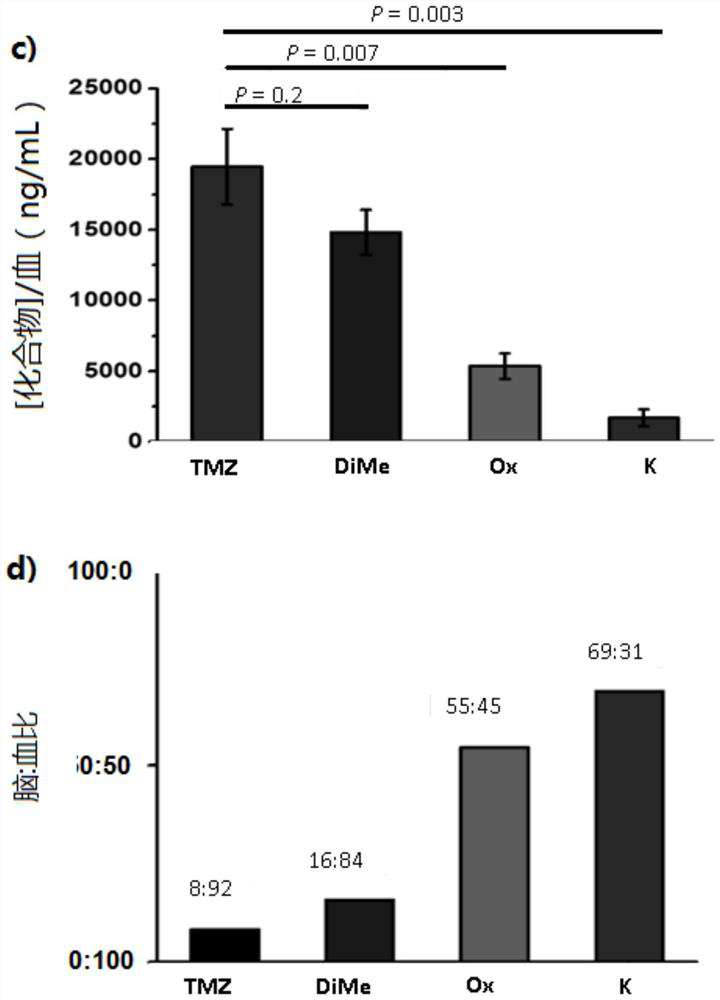
[0186] Example 1: Experimental information on biological data
[0187] Cell Culture and Reagents: All cell lines were maintained at 37°C, 5% CO 2 and a humid environment in a medium containing 1% penicillin / streptomycin. Cell culture conditions were as follows: Traditional cell lines U87 and T98G were grown in EMEM with 10% FBS. Traditional cell lines D54 and U118MG were grown in DMEM with 10% FBS. HGCC patient-derived cell line U3054MG 1 Cultured under serum-free stem cell conditions (1:1 Neurobasal:DMEM / F12 medium supplemented with B27, N2, hEGF and hFGF). GBM hookococcus cell line Br23c 2 Cultures were performed with NeuroCult NS-A proliferation kit (Stem Cell Technologies) supplemented with 0.0002% heparin, hEGF and hFGF. Temozolomide (TMZ) was purchased from AK Scientific. TMZ analogs were synthesized as described below. Compounds were dissolved in DMSO (1% final concentration, Fisher Chemical) for cell culture studies.
Embodiment 2
[0194] Embodiment 2: synthetic method
[0195] Materials and methods: Chemical reagents were purchased from commercial sources and used without further purification. Flash chromatography uses silica gel (230-400 mesh). The anhydrous solvent was dried by passing it through a column packed with activated alumina under a positive pressure of nitrogen. All reactions were performed in oven-dried glassware under a nitrogen atmosphere with magnetic stirring unless otherwise stated. 1 H and 13 C NMR spectra were recorded on a Bruker 500 (500MHz, 1 H; 125MHz, 13 C) or Varian Unity Inova 500 (500MHz, 1 H) MHz spectrometer. Spectra were referenced using residual chloroform (δ = 7.26ppm, 1 H; 77.16ppm, 13 C) or dimethyl sulfoxide (δ=2.50ppm, 1 H; 39.52ppm, 13 C). Multiplicity is expressed as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) and br (broad). The coupling constant J is expressed in Hertz (Hz). High resolution mass spectrometry (HRMS) was performe...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap