Application of pyrimidinedione compound as microsome prostaglandin E2 synthase inhibitor
A technology of synthase inhibitors and pyrimidine diketones, which is applied in the application field of pyrimidine diketones as microsomal prostaglandin E2 synthase inhibitors, can solve the side effects of cardiovascular diseases and other problems, and achieve less adverse reactions and high efficacy. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Experimental procedure
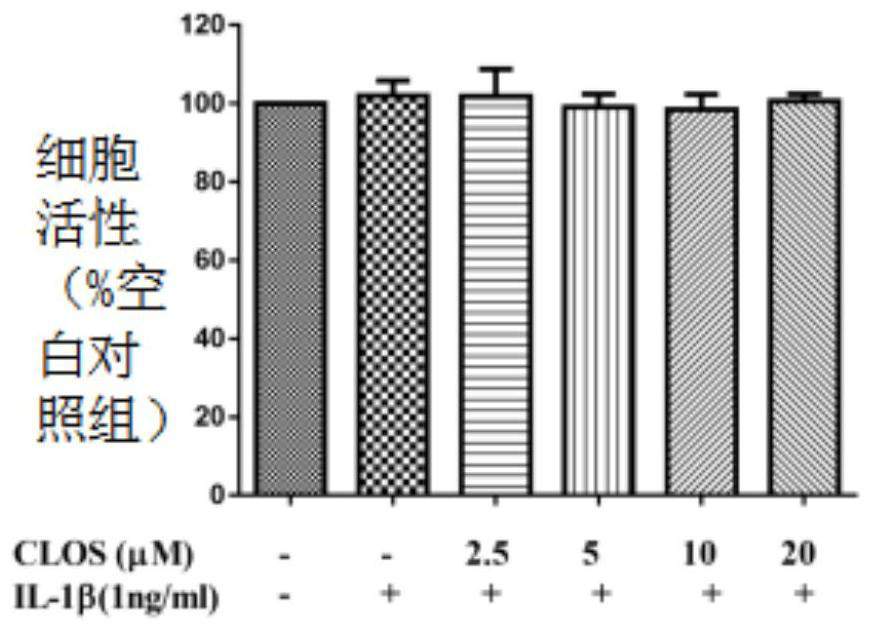
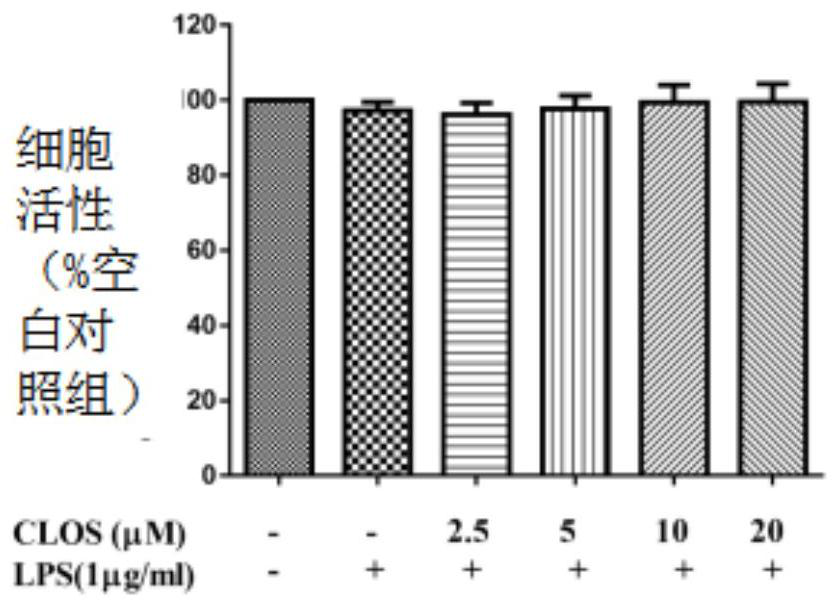
[0059] (1) A549 cell culture and treatment
[0060] A549 cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA).
[0061] The cell culture medium is RPMI 1640, and 10% fetal bovine serum (Gibco BRL Co, Grand Island, NY, USA) and 1% penicillin G (100ml / unit), streptomycin (100mg / ml) and L- Glutamine (2 mM) (GibcoBRL Co, Grand Island, NY, USA).
[0062] Cells at 37°C with 5% CO 2 Incubate in an environment; A549 cells were incubated at 1×10 5 seeded in 6-well plates and incubated for 24 hours.
[0063] (2) Isolation and culture of primary rat peritoneal macrophages
[0064] SD rats (body weight 180-280g) purchased from the University of Hong Kong were provided, and peritoneal macrophages (RPM) were isolated from the mice and cultured in Dulbecco's modified Eagle's medium (DMEM); wherein, the medium was supplemented with 10% Fetal bovine serum (Gibco BRL Co, Grand Island, NY, U...
Embodiment 2
[0071] CLOS versus PGE in cell-free ELISA assay 2 and mPGES-1 protein activity
[0072] Experimental procedure
[0073] (1) Preparation of mPGES-1 protein
[0074] A549 cells were treated with 1.6×10 6 The density is inoculated at 175cm 2 and incubated for 24 hours; then stimulated the cells with 1ng / mL IL-1β for 72 hours; collected the cells and washed them twice with PBS; centrifuged the collected cell pellet at 1000rpm for 5 minutes, then discarded the supernatant; Pre-cooled homogeneous buffer (0.1M potassium phosphate buffer pH7.4, 1mM phenylmethanesulfonyl fluoride, 60μg / mL soybean trypsin inhibitor, 1μg / mL leupeptin, 2.5mM GSH and 250mM sucrose) weight Suspended cell pellets were disrupted with a sonic oscillator. The suspension was collected and centrifuged at 10000g for 10 minutes. The supernatant was collected and centrifuged at 50000 rpm for 1.5 hours. After ultracentrifugation, the supernatant was discarded, the pellet was collected and resuspended in homo...
Embodiment 3
[0080] Affinity detection of mPGES-1 protein and compounds in a cell-free assay
[0081] Experimental procedure
[0082] Assays were performed using a FortéBio Octet Red instrument, and all assays were performed using 96-well plates (Greiner Bio-One, PN: 655209). All final reaction volumes were controlled at 200 μL / well. Biotinylated mPGES-1 protein was immobilized on SA tips. The assay steps include: baseline zeroing, compound binding, and dissociation. Analysis of protein and drug affinity results was performed by ForteìBio data analysis software. To measure the interaction between compound CLOS and mPGES-1 protein, seven concentrations of compound CLOS (3.13, 6.25, 12.5, 25, 50, 100 μΜ) were employed in this assay.
[0083] Result analysis
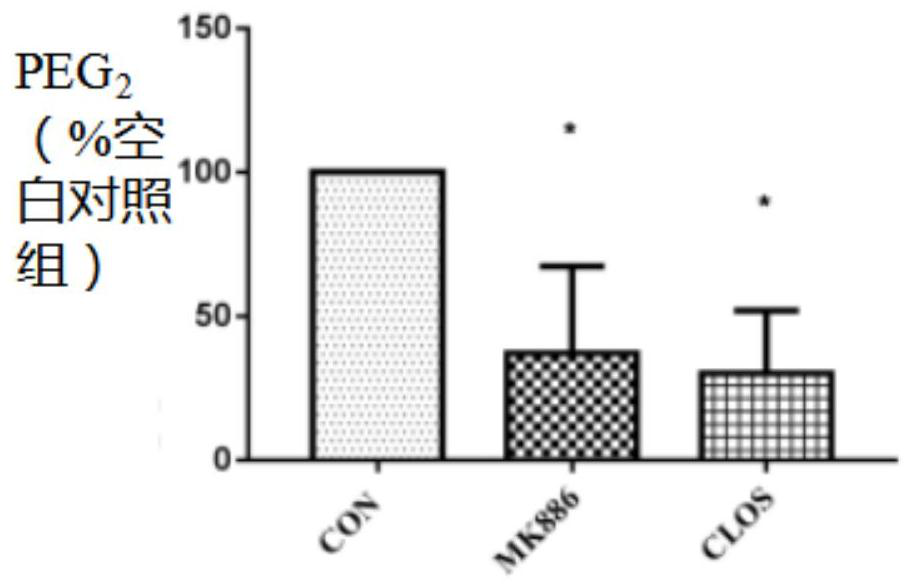
[0084] Since the results of Example 2 have shown that CLOS can simultaneously reduce PGE 2 and the protein activity of mPGES-1, so the molecular docking method was used in this study to test the binding ability of CLOS to mPGE...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com