Conjugates and nanoparticles of hyaluronic acid and epigallocatechin-3-O-gallate and uses thereof
A technology of epigallocatechin and gallocatechin, applied to the conjugates and nanoparticles of hyaluronic acid and epigallocatechin-3-O-gallate and the fields of their uses, can solve the limited availability , lack of specificity and efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
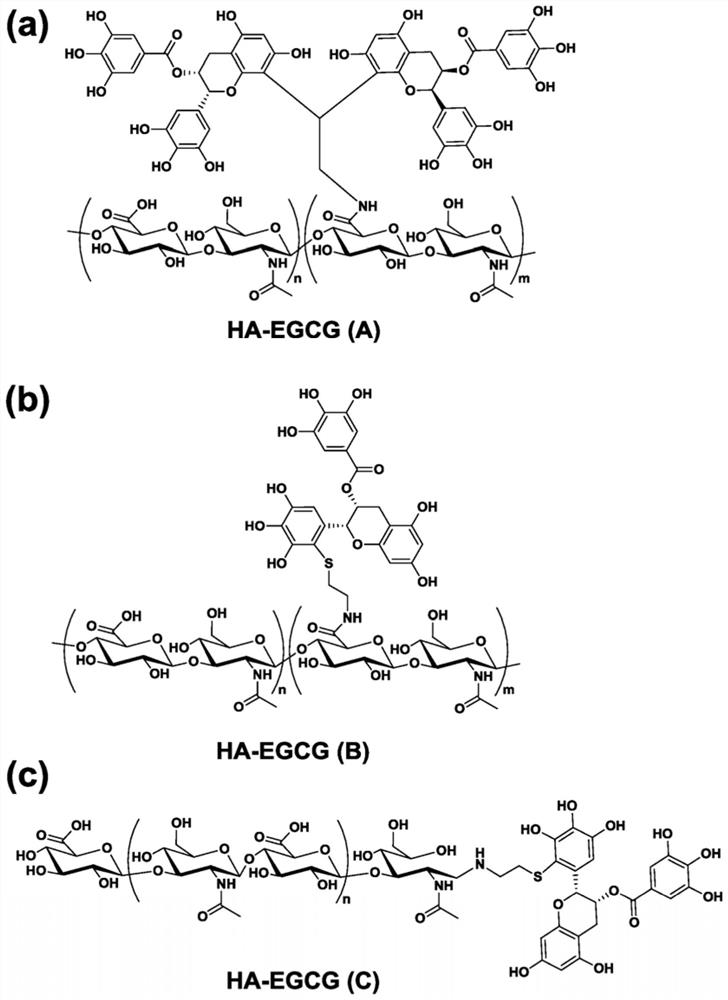
[0183] Example 1. Preparation of HA-EGCG nanoparticles comprising small molecule inhibitors
[0184] One of the HA-EGCG conjugates (A)-(C) with FLT3 inhibitor was used to synthesize the HA-EGCG nanoparticles of the present invention. Sunitinib and sorafenib were selected as representative type I and type II FLT3 inhibitors, respectively, to prepare these nanoparticles. Sunitinib is a type I inhibitor that blocks FLT3 signaling by binding to its intracellular ATP-binding site when the receptor is in the active conformation, while sorafenib blocks FLT3 signaling when the receptor is inactivated Type II inhibitors that bind to hydrophobic regions near the only accessible ATP-binding site (M. Larrosa-Garcia, M.R. Baer, Mol. Cancer Ther. 2017, 16, 991-1001).
[0185] Unlike type II inhibitors, which can only kill AML cells with FLT3-ITD mutations, type I inhibitors can be used to treat AML cells with FLT3-ITD mutations as well as those with FLT3 tyrosine kinase domain (TKD) muta...
Embodiment 2
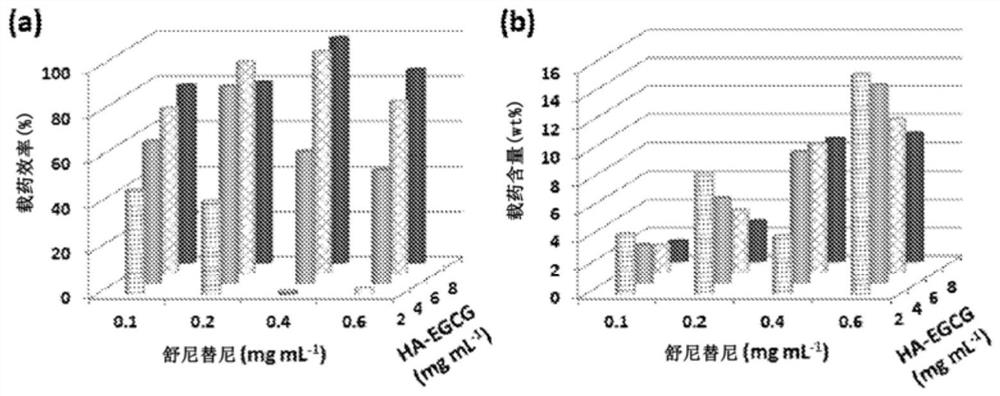
[0200] Example 2. Characterization of HA-EGCG nanoparticles comprising sunitinib or sorafenib
[0201] The prepared HA-EGCG nanoparticles of Example 1 were characterized by dynamic light scattering to determine the hydrodynamic size of the particles. In addition, the drug loading efficiency and drug loading content of the nanoparticles were determined.
[0202] experiment
[0203] dynamic light scattering
[0204] The hydrodynamic diameter of the nanoparticles was examined by dynamic light scattering using a Nano ZS zetasizer (Malvern Instruments, UK). All measurements were performed in triplicate at 37°C.
[0205] Drug loading efficiency and drug loading content
[0206] To examine the loading of sunitinib in nanoparticles, each sample was diluted 50-fold in 25% ethanol-water solution and the absorbance at 431 nm was measured on a Hitachi U-2810 spectrophotometer. Various concentrations of sunitinib malate (1-10 μg mL -1 ) to build a calibration curve.
[0207] The...
Embodiment 3
[0225] Example 3. In vitro anti-leukemia activity of prepared HA-EGCG nanoparticles comprising sunitinib or sorafenib
[0226] The in vitro anti-leukemic activity of selected HA-EGCG nanoparticles comprising sunitinib or sorafenib (in Example 2) was evaluated on MOLM-14 and MV-4-11 cells.
[0227] experiment
[0228] MOLM-14 and MV-4-11 cells (ATCC, USA) were maintained in RPMI 1640 culture supplemented with 10% (v / v) fetal bovine serum (FBS) and 1% (v / v) penicillin / streptomycin Base. Cells (1×10 4 cells / well) were incubated in 100 μL of 10% FBS-supplemented medium containing various concentrations of sunitinib or sorafenib-loaded nanoparticles and the respective free drug. In the case of free sorafenib, a stock solution of sorafenib tosylate was prepared in dimethyl sulfoxide (DMSO), and then diluted to a final DMSO concentration of 1% with RPMI 1640 medium; this for DMSO Both concentrations had no detectable effect on leukemia cell growth. After 3 days of treatment, 100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com