Synthesis method of (S)-nicotine
A synthesis method and technology of nicotine, applied in the direction of organic chemistry, fermentation, etc., can solve the problems of non-recovery, no practical application value, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
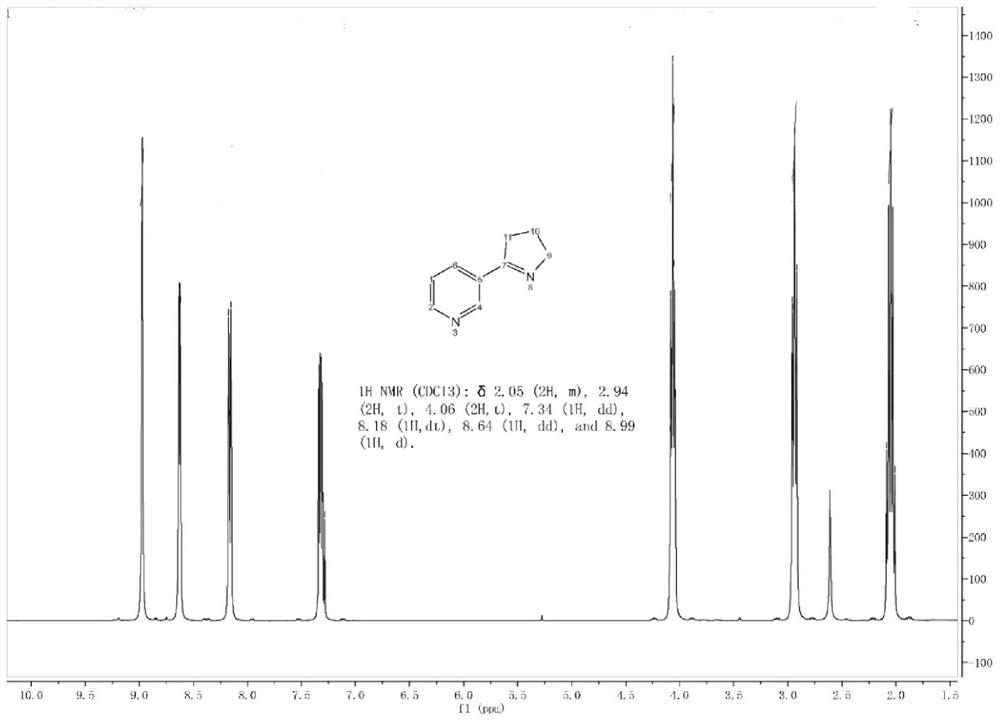
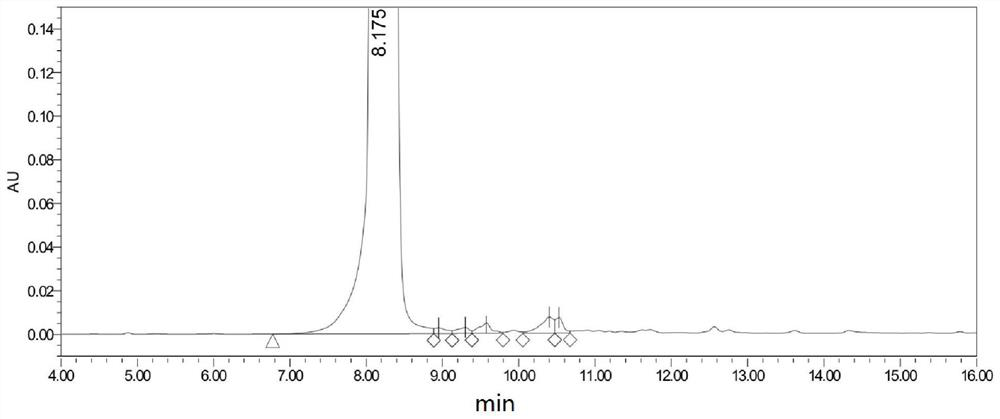
[0059] This example prepares the compound Mesmin, and its synthesis method is carried out with reference to the method in the patent document EP2487172A1, and the obtained product is characterized by proton nuclear magnetic spectrum, and the characterization diagram is as follows figure 1 As shown, the NMR data results are: 1 H-NMR (400MHz, CDCl 3 ): δppm 8.97(1H,d),8.64(1H,dd),8.17(1H,dt),7.29-7.34(m,1H),4.06(2H),2.91-2.96(m,2H),2.01-2.09 (m,2H). It indicated that mesmin was synthesized successfully. The sample is analyzed and detected by high-performance liquid chromatography, and its purity is 99.3%, and the chromatographic characterization diagram is as follows figure 2 shown.
Embodiment 2
[0061] This embodiment provides a synthetic method of (S)-nornicotine, the operation method is as follows:
[0062] Add 3 g of mesmin into a 50 mL three-neck round bottom flask, add 10 mL of 0.1 M phosphate buffer, and adjust the pH to 6.0. Then add 5.5 g of glucose into the reaction flask and stir until completely dissolved. In another 50mL flask, add 0.2g imine reductase IRED103, 0.04g glucose dehydrogenase GDH109 and 0.008g NADP salt, stir until completely dissolved. Then the solution in the second flask was slowly added to the first flask, the temperature was raised to 25°C, and the reaction was stirred at 300r / min for 24h. After filtration, the filtrate was extracted with chloroform, dried over anhydrous sodium sulfate, and concentrated to obtain 2.2 g of (S)-nornicotine.
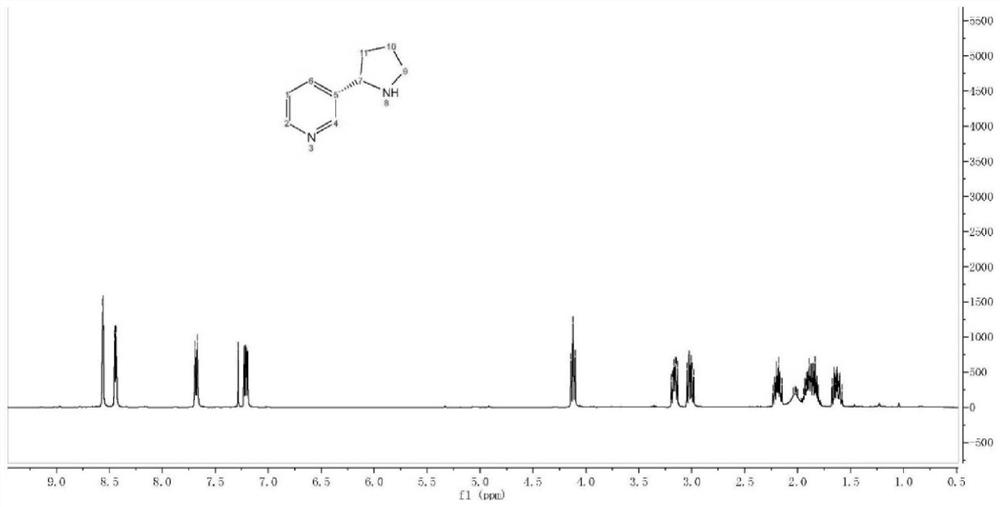
[0063] The prepared (S)-nornicotine is characterized by proton nuclear magnetic spectrum, and the characterization diagram is as follows image 3 As shown, the NMR data results are: 1 H-NMR (400MHz...
Embodiment 3
[0066] This embodiment provides a synthetic method of (S)-nornicotine, the operation method is as follows:
[0067] Add 3 g of mesmin to a 50 mL three-neck round bottom flask, add 15 mL of 0.1 M phosphate buffer, and adjust the pH to 5.8. Then add 5.5 g of glucose into the reaction flask and stir until completely dissolved. In another 50mL flask, add 0.3g imine reductase IRED103, 0.04g glucose dehydrogenase GDH102 and 0.01g NADP salt, stir until completely dissolved. Then slowly add the solution in the second flask to the first flask, raise the temperature to 37°C, and stir for 24h. After filtration, the filtrate was extracted with chloroform, dried over anhydrous sodium sulfate, and concentrated to obtain 2.4 g of (S)-nornicotine.
[0068] Utilize high-performance liquid chromatography to carry out the mensuration of chemical purity and optical purity to the (S)-nornicotine that makes respectively, concrete operation method is the same as embodiment 2, and chromatogram is a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com