L-cepharanthine sustained-release microsphere and preparation method thereof
A technology of levothrinine and sustained-release microspheres, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., and can solve the problems of poor oral absorption and low bioavailability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
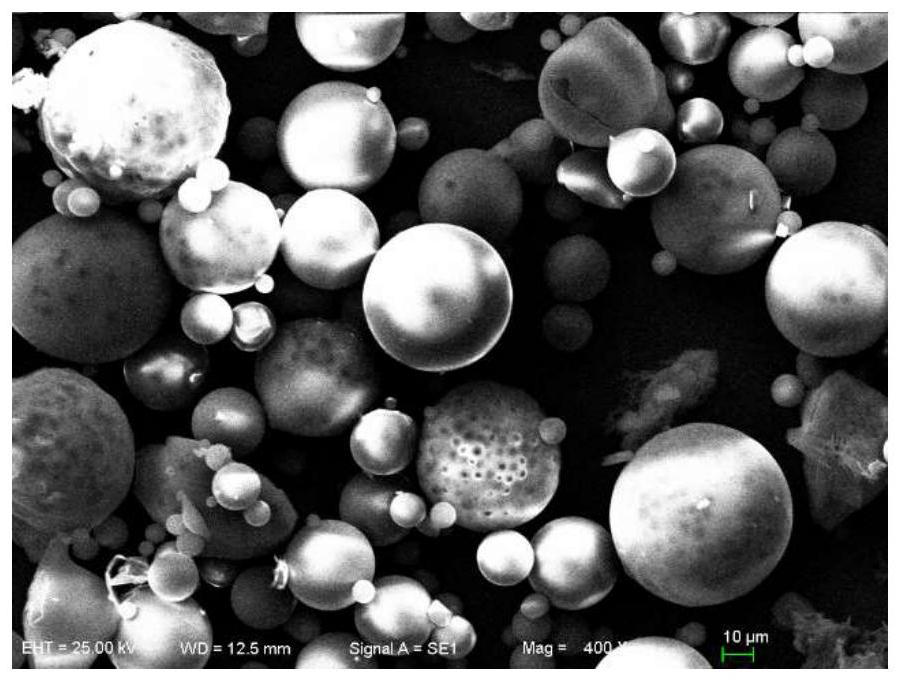
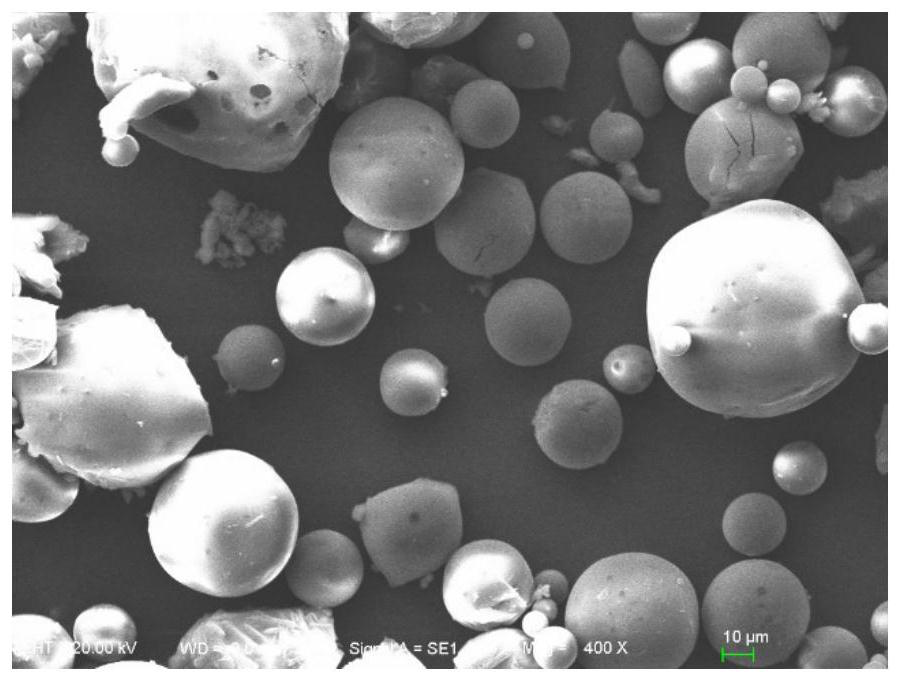
Image
Examples
Embodiment 1
[0038] Weigh 100 mg of PLGA (molar ratio of lactide to glycolide = 75:25, Mw = 1.2wg / mol), dissolve it in 0.5 mL of dichloromethane, weigh 50 mg of L-steprenine, add the drug Ultrasonic pulverize (200W, 2s, 45s) into the dichloromethane solution of PLGA to disperse evenly. Drop by drop into 2.5mL of 1% PVA solution under stirring and emulsify quickly using a vortex device, then add it into 62.5mL of water under stirring to solidify the microspheres and volatilize the organic solvent, and collect the microspheres after 2 hours The balls were washed with water for 3-5 times, the suspension was placed in a -80°C refrigerator for 8 hours, and then freeze-dried for 48 hours to obtain microsphere powder.
Embodiment 2
[0040] Weigh 100mg of PLGA (molar ratio of lactide to glycolide = 75:25, Mw = 1.2wg / mol), dissolve in 5mL of dichloromethane, weigh 50mg of L-stephenidine, dissolve in 0.5mL Add the DMSO solution containing the drug to the dichloromethane solution of PLGA, vortex to disperse evenly, and drop it into 2.5mL of 1% PVA solution under stirring to form an O / W emulsion . Stirring was continued at room temperature for 3 h to volatilize dichloromethane, wherein the stirring rate was 300-500 rpm. After evaporating the organic solvent, add the above system into 625mL of water to solidify for 4 hours, collect the microspheres and wash them with water for 3-5 times, suspend them in a -80°C refrigerator for 8 hours, freeze-dry for 48 hours, and obtain microsphere powder .
Embodiment 3
[0042]Weigh 100mg of PLGA (molar ratio of lactide to glycolide = 75:25, Mw = 1.2wg / mol), dissolve in 5mL of dichloromethane, weigh 20mg of L-paternidine, dissolve in 0.5mL In DMSO, the DMSO solution containing the drug was added to the dichloromethane solution of PLGA, and vortexed to disperse evenly. It was added dropwise into 25 mL of 1% PVA solution under stirring to form an O / W emulsion. Stirring was continued at room temperature for 3 h to volatilize dichloromethane, wherein the stirring rate was 300-500 rpm. After evaporating the organic solvent, add the above system into 625mL of water to solidify for 4 hours, collect the microspheres and wash them with water for 3-5 times, suspend them in a -80°C refrigerator for 8 hours, freeze-dry for 48 hours, and obtain microsphere powder .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com