Medicament for infantile autism caused by hypoxic-ischemic brain injury and preparation method thereof
A technology for hypoxia-ischemia and brain injury, which can be applied to medical formulas, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as insignificant efficacy of brain injury functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
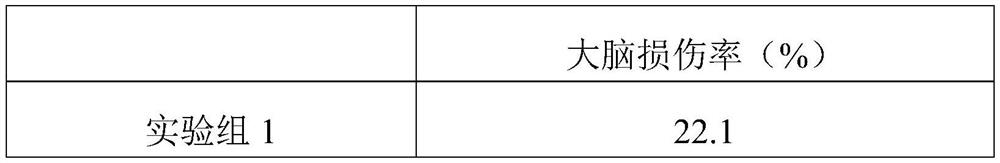
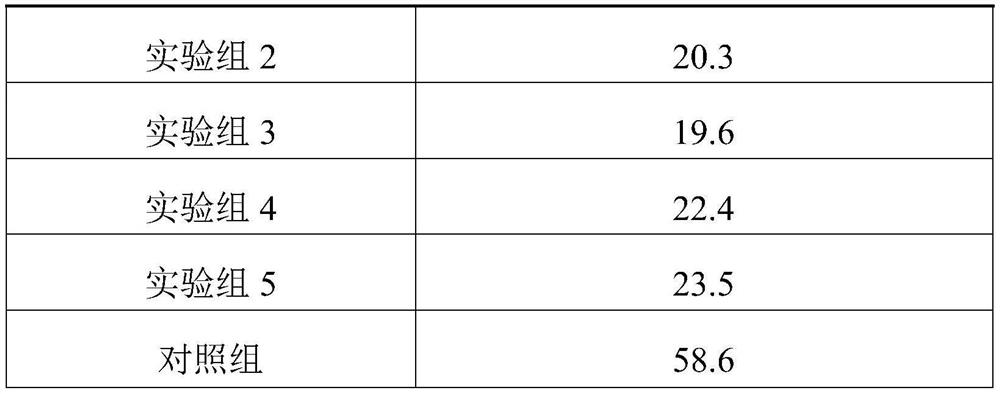
[0021] A medicament for children with autism caused by hypoxic-ischemic brain injury, comprising the following raw materials in parts by weight: 20 parts of ganoderma acid derivatives, 18 parts of total triterpenes of ganoderma lucidum, 26 parts of ligustrazine hydrochloride, 22 parts of bilobalide, 10 parts of teretin B, 12 parts of luteolin, 3 parts of pyridoxine.
Embodiment 2
[0023] A medicament for children with autism caused by hypoxic-ischemic brain injury, comprising the following raw materials in parts by weight: 50 parts of ganoderma acid derivatives, 45 parts of total triterpenes of ganoderma lucidum, 30 parts of ligustrazine hydrochloride, 34 parts of bilobalide, 20 parts of teretin B, 22 parts of luteolin, 15 parts of pyridoxine.
Embodiment 3
[0025] A medicament for children with autism caused by hypoxic-ischemic brain injury, comprising the following raw materials in parts by weight: 30 parts of ganoderma acid derivatives, 35 parts of total triterpenoids of ganoderma lucidum, 28 parts of ligustrazine hydrochloride, 28 parts of bilobalide, 15 parts of luteolin, 17 parts of luteolin, and 10 parts of pyridoxine.
[0026] The raw materials in parts by weight of the above-mentioned embodiments 1-3 are prepared by the following preparation method:
[0027] Step (1): dissolving ligustrazine hydrochloride, bilobalide, teretin, and luteolin in ethanol with a mass fraction of 85%, mixing and stirring to prepare a suspension with a drug concentration of 150 mg / mL;
[0028] Step (2): Dissolving ganoderma acid derivatives, ganoderma total triterpenes, and pyridoxine in water, stirring evenly, the stirring rate is 400 rpm, and the stirring time is 100 s;
[0029] Step (3): Mix steps (1) and (2), add 3 times the volume of water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com