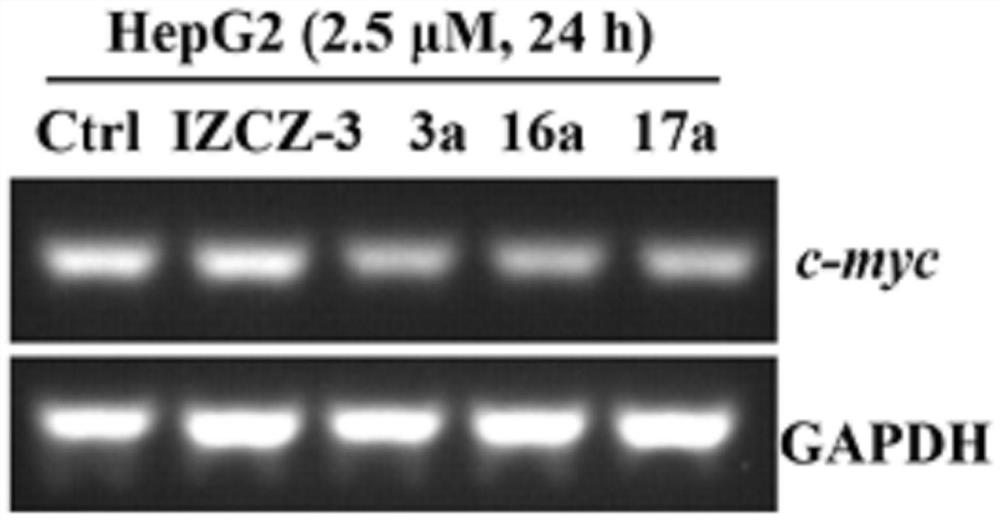
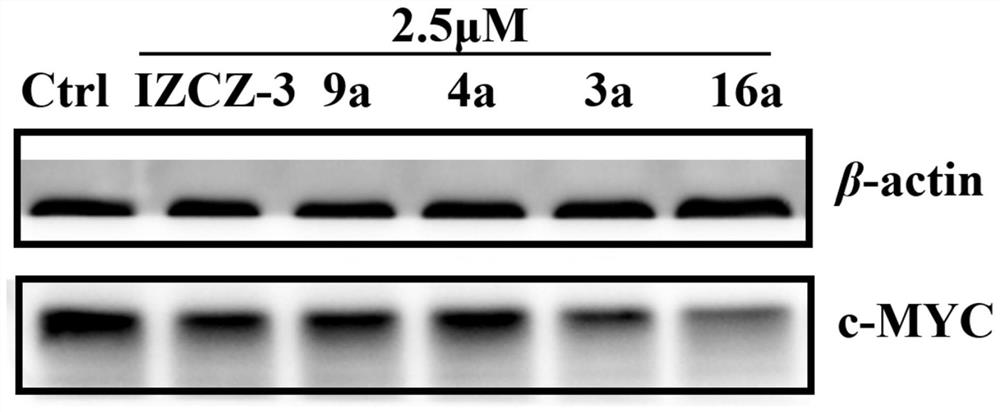
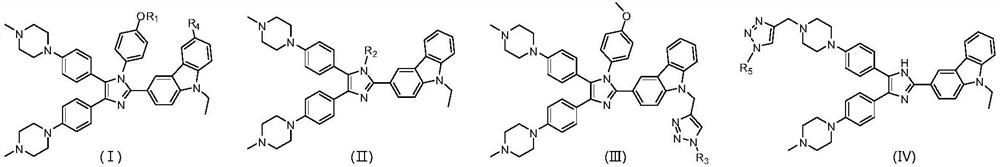
Polyaryl substituted imidazole derivative as well as preparation method and application thereof
A technology of imidazole derivatives and derivatives, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of complex transcription regulation of c-MYC gene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation of embodiment intermediate:
[0064] (1) Preparation of Compound C1-1
[0065] Difluorobenzil (490mg, 2mmol) and potassium carbonate (829mg, 6mmol) were dissolved in 6mL DMF, and N-methylpiperazine (1g, 10mmol) was added dropwise. The mixture was reacted overnight at 80°C. After the reaction, the reaction solution was poured into ice water, extracted with ethyl acetate, washed with saturated brine, concentrated and dried, separated and purified by column chromatography to obtain compound C1-1 (818 mg, 99%) as a yellow solid, 1 H NMR (400MHz, CDCl 3 )δ7.85(d, J=9.0Hz, 4H), 6.85(d, J=9.1Hz, 4H), 3.41(t, J=5.2Hz, 8H), 2.54(t, J=5.1Hz, 8H) ,2.34(s,6H).
[0066] (2) Preparation of Compound D1-1
[0067] Difluorobenzil (490mg, 2mmol) and potassium carbonate (829mg, 6mmol) were dissolved in 6mL DMF, and N-methylpiperazine (300mg, 3mmol) was added dropwise. The mixture was reacted overnight at 80°C. After the reaction, the reaction solution was poured int...
Embodiment 1
[0081] Preparation of compound 1a
[0082] Compound 1a was synthesized by general method C. Pale yellow solid (98% yield). 1 H NMR (400MHz, CDCl 3 )δ8.21(s, 1H), 7.93(d, J=7.7Hz, 1H), 7.56(d, J=8.4Hz, 2H), 7.44(dd, J=12.8, 8.1Hz, 2H), 7.37( d,J=8.1Hz,1H),7.23(d,J=8.7Hz,1H),7.18(t,J=7.4Hz,1H),7.00(dd,J=14.1,8.5Hz,4H),6.84( d, J=8.4Hz, 2H), 6.75(t, J=8.9Hz, 4H), 4.31(q, J=7.2Hz, 2H), 4.05(t, J=5.9Hz, 2H), 3.83(t, J=5.9Hz, 2H), 3.25(q, J=5.0Hz, 8H), 2.66(dt, J=13.2, 4.9Hz, 8H), 2.41(s, 3H), 2.39(s, 3H), 2.00( p,J=5.9Hz,2H),1.40(t,J=7.2Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ158.2,150.1,149.4,140.4,139.7,137.6,132.1,130.8,129.9,129.8,128.3,127.0,125.9,123.2,122.8,122.2,121.7,121.7,120.7,119.1,116.0,115.5,114.8,108.7,108.1 ,65.7,60.2,55.0,54.9,48.8,48.3,45.9,45.8,37.7,32.1,14.0.HRMS(ESI)m / z: calcd for C 48 h 53 N 7 o 2 :380.7203[M+2H] 2+ .Found 380.7203[M+2H] 2+ .
Embodiment 2
[0084] Preparation of compound 2a
[0085] Compound 2a was synthesized by general method A. Pale yellow solid (72% yield). 1 H NMR (500MHz, CDCl 3 )δ8.21(s,1H),7.93(d,J=7.8Hz,1H),7.57(d,J=8.3Hz,2H),7.50–7.40(m,2H),7.36(d,J=8.1 Hz, 1H), 7.22(d, J=8.6Hz, 1H), 7.18(t, J=7.4Hz, 1H), 7.01(dd, J=16.4, 8.3Hz, 4H), 6.84(d, J=8.4 Hz, 2H), 6.75(dd, J=18.0, 8.4Hz, 4H), 4.30(q, J=7.2Hz, 2H), 4.23(t, J=6.2Hz, 2H), 3.96(t, J=6.2 Hz, 2H), 3.21(t, J=5.0Hz, 8H), 2.56(q, J=4.9Hz, 8H), 2.34(s, 6H), 2.08(p, J=6.2Hz, 2H), 2.03( s,3H),1.39(t,J=7.2Hz,3H). 13 C NMR (125MHz, CDCl 3 )δ171.1,158.1,150.2,149.6,147.7,140.3,139.6,137.7,132.1,130.8,129.8,129.7,128.2,126.9,126.7,125.8,123.2,122.7,122.0,121.7,121.6,120.6,119.1,115.8,115.3 ,114.7,108.6,108.0,64.6,61.4,55.3,55.2,49.1,48.5,46.3,37.7,28.6,21.0,13.9.HRMS(ESI)m / z: calcd for C 50 h 55 N 7 o 3 :401.7256[M+2H] 2+ .Found 401.7253[M+2H] 2+ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com