Synthesis method of propyzamide based on oxidation reaction
A technology of propargyl and a synthesis method, which is applied in the field of organic chemical synthesis of pesticide molecules, can solve the problems of high toxicity, high safety risk, high cost of propargyl, and achieves low cost, mild reaction conditions, and high cost. The effect of commercial value and potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
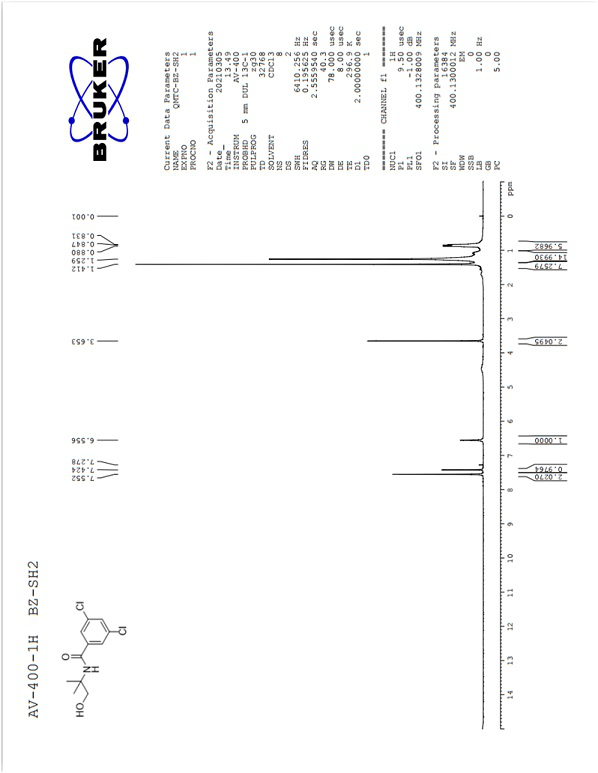
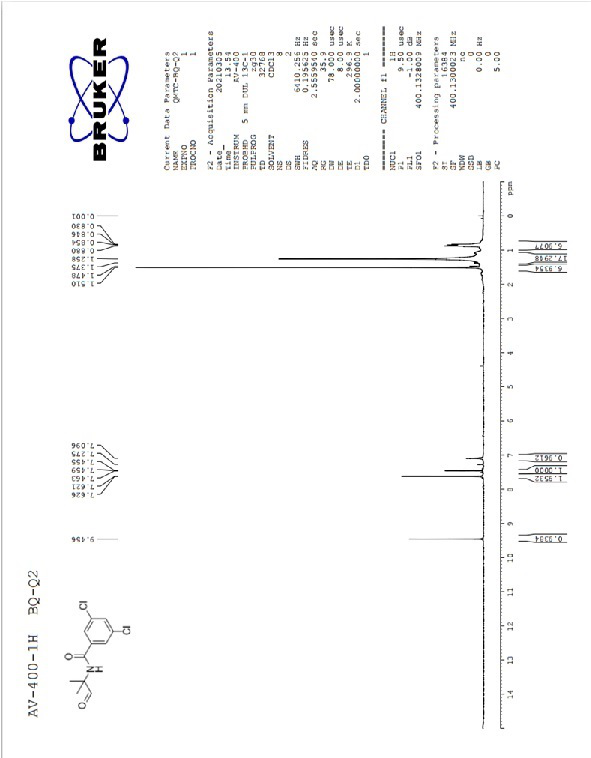
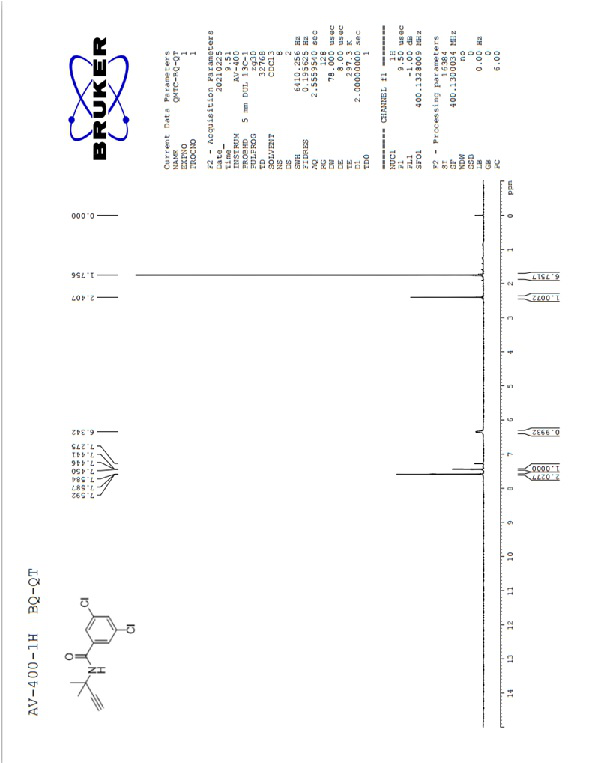
Image
Examples
Embodiment approach
[0015] According to one embodiment of the present invention, the synthesis method comprises the following steps:
[0016] S1.3,5-dichlorobenzoyl chloride is condensed with 2-amino-2 methyl 1-propanol in an organic solvent under the action of a coupling reagent to generate 3,5-dichloro-N-( 1-hydroxy-2-methylpropan-2-yl) benzamide;
[0017] S2. Use an oxidant to oxidize the 3,5-dichloro-N-(1-hydroxy-2-methylpropan-2-yl)benzamide obtained in step S1 in an organic solvent to generate 3,5-dichloro -N-(2-methyl-1-oxypropan-2-yl)benzamide;
[0018] S3. Using dimethyl (1-diazo-2-oxopropyl)phosphonate to 3,5-dichloro-N-(2-methyl-1-oxopropan-2-yl) obtained in step S2 Benzamide undergoes acetylenation reaction to generate 3,5-dichloro-N-(2-methylbut-3-yn-2-yl)benzamide, which is the product of benzamide.
[0019] In step S1, a condensation reaction is carried out in an organic solvent using a coupling reagent: the coupling reagent is N,N'-carbonyldiimidazole, dicyclohexylcarbodiimide,...
Embodiment 1
[0028]
[0029] Take an original bottom flask with a built-in stirring bar. Add 3,5-dichlorobenzoic acid (2.00 g, 10.47 mmol) and dichloromethane (20 ml) to it, stir, then add N,N'-dicarbonylimidazole (1.87 g, 11.52 mmol), stir For 30 minutes, react until the system dissolves. Add 2-amino-2-methyl-1-propanol (1.03 g, 11.52 mmol) into the reaction system and react at room temperature for 1 hour. After the reaction, water (40 ml) and dichloromethane (20 ml) were added to the reaction system, the organic phase (lower layer) was separated, and the aqueous phase was extracted three times with dichloromethane (10 ml). The organic phases were combined and concentrated to dryness to obtain the crude product of 3,5-dichloro-N-(1-hydroxy-2-methylpropan-2-yl)benzamide as light yellow oil with a yield of 89%, NMR The calculated content of the spectrum is 92%. The crude product was directly used in the next reaction.
Embodiment 2
[0031]
[0032] Take an original bottom flask with a built-in stirring bar. Add 3,5-dichloro-N-(1-hydroxy-2-methylpropan-2-yl)benzamide (2.05 g, 7.82 mmol) and dichloromethane (10 ml) to it, and stir until the system dissolves . Add Dess-Martin reagent (3.65 g, 8.60 mmol) and water (0.1 ml) to it, and react at room temperature for 1 hour. After the reaction was completed, a saturated sodium bicarbonate solution (5 ml) was added thereto and reacted for 5 minutes. The organic phase was separated, the aqueous phase was extracted three times with dichloromethane (5 ml), the combined organic phases were washed with saturated sodium thiosulfate solution (5 ml), and concentrated to dryness. 3,5-Dichloro-N-(2-methyl-1-oxypropan-2-yl)benzamide was obtained as a white solid with a yield of 95% and a purity of 95% calculated by NMR. This product was used directly in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com