Chromone oxadiazoline compound as well as preparation method and application thereof
A chromone-bioxadiazoline and bioxadiazoline technology, which is applied in the field of chromone-bioxadiazoline compounds and their preparation, can solve the problems of high cost, high toxicity, difficulty in large-scale production and the like, and achieves overcoming Effects of high toxicity and broad research prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
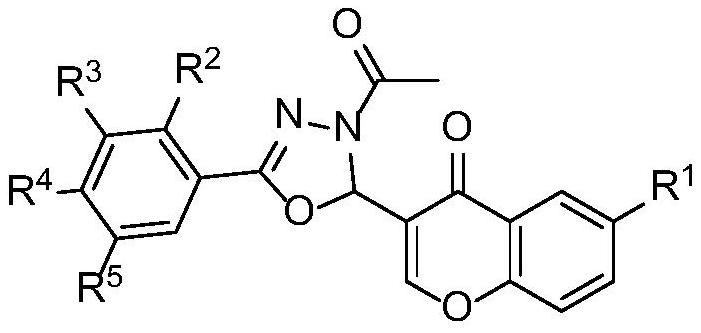
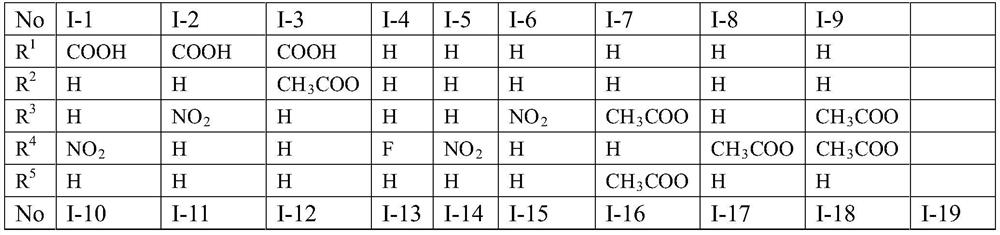
[0026] (1) Compound I-1, namely 3-(3-acetyl-5-(4-nitrophenyl)-2,3-dihydro-1,3,4-oxadiazole-2-)-4 -Oxo-4H-chromone-6-carboxylic acid, whose structural formula is as follows:
[0027]
[0028] The specific preparation process is as follows: take 381mg (1mmol) of compound II-1, add 10g (excessive) acetic anhydride, heat and reflux for more than 1h, the reaction solution changes from turbid to clear liquid; TLC monitors the reaction process until the reaction is complete; cooling, add 50mL water, stirring continuously, to completely hydrolyze acetic anhydride, a solid precipitated out, suction filtered, washed with water, dried, and separated by PE / EtOAc column chromatography to obtain the target product.
[0029] The obtained pure product is 127mg of yellow powder, the yield is 30%, the melting point is 227-231°C, and the molecular formula is C 20 h 13 N 3 o 8 , to detect it, and the detection result is: 1 H NMR (600MHz, DMSO-d 6 )δ13.62–13.24(m,1H,COOH),8.95–7.71(m,8H,=...
Embodiment 2
[0085] Cy-FBP / SBPase enzyme inhibition experiments were carried out on the 19 compounds synthesized above. Cy-FBP / SBPase can catalyze the hydrolysis of fructose-1,6-bisphosphate into fructose-6-phosphate (F6P) and inorganic phosphate (Pi). The product Pi can form a blue-green complex with the basic dye malachite green and ammonium molybdate mixture in the presence of polyhexenol, and Cy can be determined by detecting the change in the ultraviolet absorbance value of the complex at 620nM. - Enzyme activity of FBP / SBPase, and then detect the inhibitory effect of the above compounds on Cy-FBP / SBPase.
[0086] The specific experimental process is as follows:
[0087] 1. Add 50mM Tris-HCl (pH 8.0), 15mM MgCl to the reaction system 2 , 10mM DTT, an appropriate concentration of Cy-FBP / SBPase and a certain concentration gradient of the above compounds;
[0088] 2. Add 0.5mM substrate (FBP) to start the reaction, and after incubating at 37°C for 5min, stop the catalytic reaction wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com