Novel ertapenem sodium crystal form and preparation method thereof
A technology of ertapenem sodium and crystal form, which is applied in the field of organic synthesis, can solve the problems of low purity and yield, and achieve the effects of improving purity, easy production, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
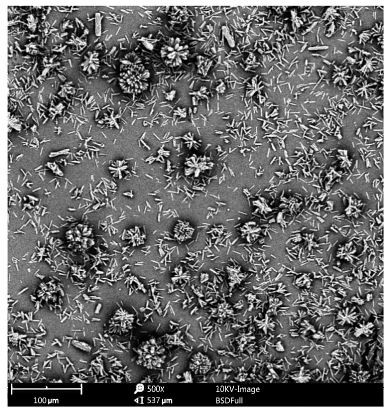
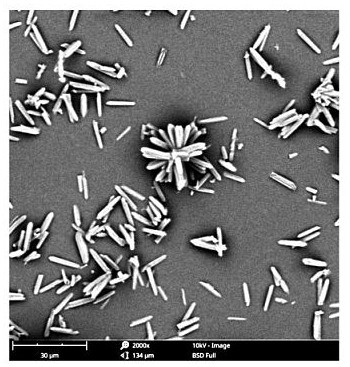
Image
Examples
preparation example Construction
[0037] The present invention also provides a method for preparing the above-mentioned new crystal form of ertapenem sodium, the preparation method comprising the following steps: adding crude ertapenem sodium to an aqueous alkali solution containing sodium ions to form a first system; Add methanol and n-propanol to the first system to form the second system; after cooling the second system to -10~15°C, add the mixed solution of acid, methanol and n-propanol to the second system in 3~10 times, The third system is formed; the volume of the mixed solution is 30~50% of the volume of the second system; after the temperature of the third system is lowered to -30~-15°C, the temperature of the third system is raised to -10~15°C to form the third system Four systems; the fourth system is sequentially filtered, washed and dried to obtain a new crystal form of ertapenem sodium; wherein, the HPLC purity of the crude ertapenem sodium is 90-98%.
[0038] The present invention can effectivel...
Embodiment 1
[0055] The preparation process is as follows:
[0056] Sodium bicarbonate solution (purified water 1000mL, sodium bicarbonate 25.35g) was prepared, and 100g of crude ertapenem sodium (CAS: 153773-82-1; HPLC purity 93%) was added to it to form the first system. The concentration of crude ertapenem sodium in the first system was 100 mg / mL.
[0057] Methanol (280ml) and n-propanol (350ml) were added to the first system to form a second system.
[0058] At 0~5°C, add the mixed solution of acetic acid, methanol and n-propanol (acetic acid: 18.15g, methanol 250ml, n-propanol 300ml) to the second system in 8 times to form the third system. The amount of each addition is 12.5% of the volume of the mixed solution of acetic acid, methanol and n-propanol; the time interval between two adjacent additions is 30 minutes.
[0059] Methanol (1260ml) and n-propanol (2265ml) were added to the third system.
[0060] The temperature of the third system was lowered to -25°C, and then the temp...
Embodiment 2
[0072] The difference with Example 1 is: sodium bicarbonate consumption is 20.28g; Add acetic acid, the mixed solution of methyl alcohol and n-propanol (acetic acid: 14.52g, methyl alcohol 250ml, n-propanol 300ml) in the second system in 3 times; The temperature of the third system was lowered to -25°C, and then the temperature of the third system was raised to 5°C to obtain the fourth system. The concentration of ertapenem sodium in the first system was 100 mg / mL. The amount of each addition is 33% of the volume of the mixed solution of acid, methanol and n-propanol; the time interval between two adjacent additions is 30min. The cooling rate is 0.12°C / min, the heating rate is 0.12°C / min, and the heating and cooling cycle is only performed once.
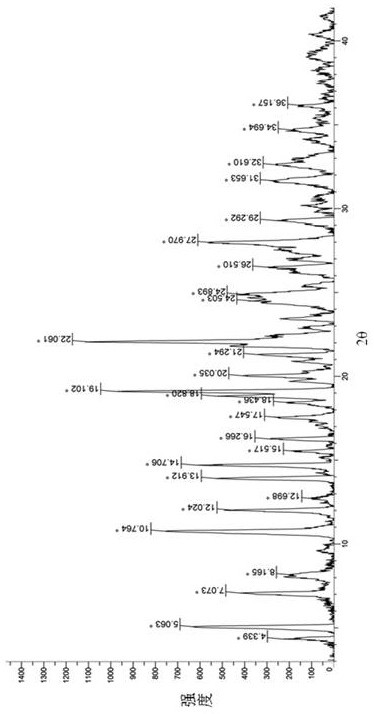
[0073] For X-ray powder diffraction (XRPD) of the above products see Figure 4 . Its crystal form has 27 main characteristic peaks ( ) at 4.4°, 5.1°, 7.1°, 8.2°, 10.8°, 12.1°, 12.8°, 14.0°, 14.8°, 15.7°, 16.4°, 17.7°, 18.6°, 19....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com