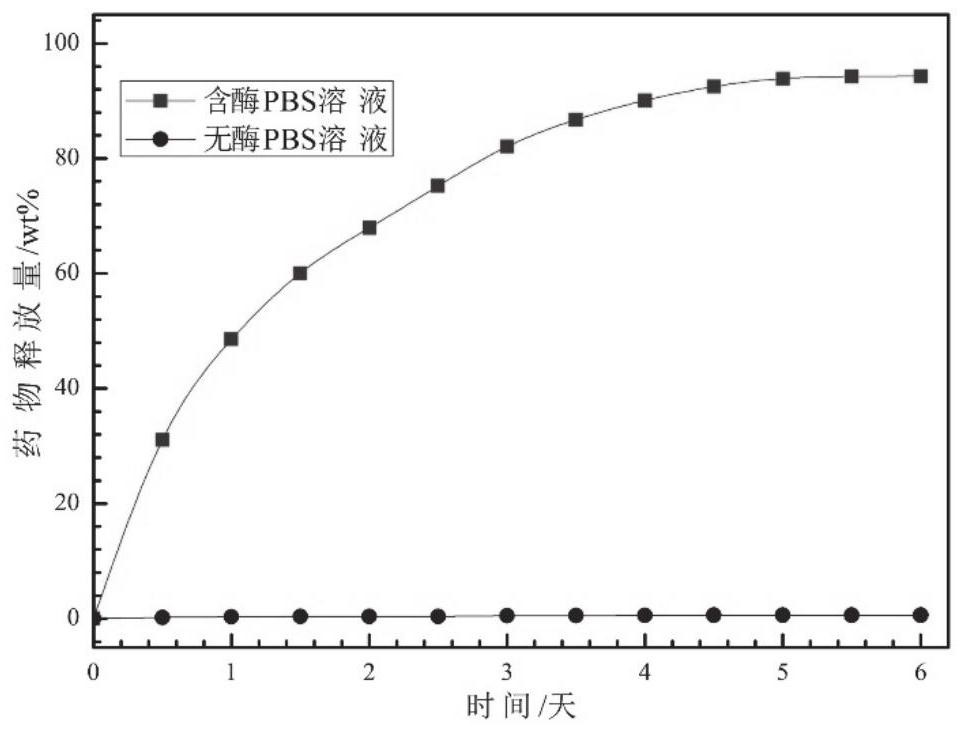
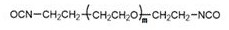A kind of hydrogel that can be loaded with high hydrophobicity drug and its preparation method and application
A hydrogel and reaction technology, applied in the field of hydrogel that can load highly hydrophobic drugs and its preparation, can solve incompatibility and other problems, and achieve the effect of easy separation, mild reaction conditions and stable ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
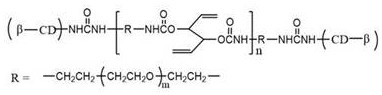
[0039] In another specific embodiment of the present invention, there is provided a preparation method of the above-mentioned cyclodextrin-terminated side chain double bond-containing polyurethane (DPU), the preparation method comprising:
[0040] The isocyanato-terminated polyethylene glycol is first reacted with 1,5-hexadiene-3,4-diol to prepare an isocyanato-terminated polyurethane prepolymer containing double bonds in the side chain, and then reacted with mono-6- O-amino-β-cyclodextrin reaction is carried out to obtain DPU;
[0041] Wherein, the molecular structural formula of isocyanato-terminated polyethylene glycol is as follows:
[0042]
[0043] In another specific embodiment of the present invention, the molecular weight of the above-mentioned isocyanato-terminated polyethylene glycol is controlled to be 3000-12000;
[0044] In yet another specific embodiment of the present invention, the molar ratio of 1,5-hexadiene-3,4-diol to isocyanato-terminated polyethylene g...
Embodiment 1
[0074] Preparation of polyurethane: 90.00 g of double-ended isocyanato polyethylene glycol (molecular weight 3000 g / mol), 1.71 g of 1,5-hexadiene-3,4-diol and 0.18 g of stannous octoate were dissolved in 200 mL of N , N-dimethylformamide (DMF), the oil bath is heated to 75 ℃ to a constant temperature reaction, until the -NCO content measured by the di-n-butylamine method reaches the theoretical value, about 3.5h. After cooling to 15°C, 34.02 g of mono-6-O-amino-β-cyclodextrin was added, and the reaction was maintained at the temperature until the infrared absorption peak of isocyanate in the detection system disappeared, about 2 hours. After the reaction is completed, wait for the system to return to room temperature, add DMF solution to dilute the cyclodextrin-terminated side chain double bond-containing polyurethane to 0.05g / mL, and then use ten times the volume of glacial ether (-6-1 ℃) for sedimentation, Suction filtration, vacuum drying at room temperature to constant wei...
Embodiment 2
[0078] Preparation of polyurethane: 90 g of double-ended isocyanato polyethylene glycol (molecular weight of 3000 g / mol), 2.05 g of 1,5-hexadiene-3,4-diol and 0.18 g of diisobutyltin dilaurel were dissolved in 200 mL In N,N-dimethylformamide (DMF), the oil bath was heated to 75°C for a constant temperature reaction, and the -NCO content was determined by the di-n-butylamine method to reach the theoretical value, about 3.5h. After cooling to 15 °C, 27.22 g of mono-6-O-amino-β-cyclodextrin was added, and the reaction was maintained at the temperature until the infrared absorption peak of isocyanate in the detection system disappeared, about 2 h. After the reaction is completed, the system is returned to room temperature, and DMF solution is added to dilute the cyclodextrin-terminated side chain double bond-containing polyurethane to 0.05 g / mL, and then settle with ten times the volume of glacial ether (-6-1 °C), pump filter, and vacuum-dry to constant weight at room temperature ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com