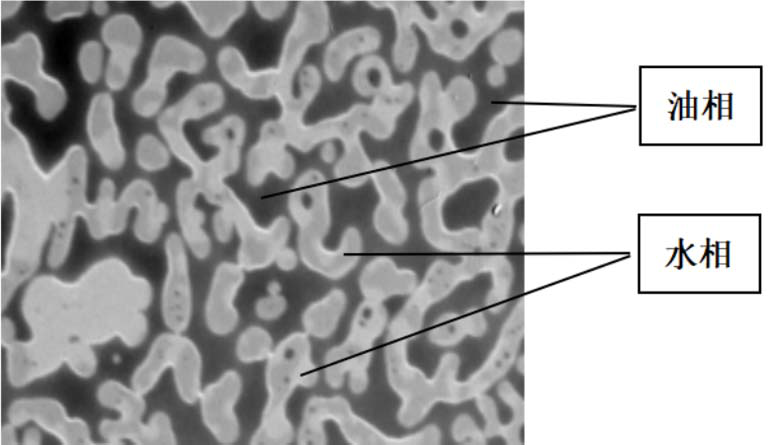
Triazaamidine sustained-release injection and preparation method thereof
A technology of sustained-release injections and triazamidine, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc. The effect duration is not long, etc., to achieve the effect of facilitating production transformation, facilitating popularization and application, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0023] In order to make the description concise, the weight composition of a triazamidine sustained-release injection described in Examples 1-10 is given in the form of a table below, as shown in Table 1 for details.
[0024] Each component of table 1 embodiment 1-10 is made up (total 100ml capacity)
[0025]
[0026] Embodiment 1-10 A preparation method of triazamidine sustained-release injection, comprising the following steps:
[0027] a) Mix the surfactant and oil in the formulated amount, heat to 38-50°C, and the system turns into a liquid;
[0028] b) Add triazine, stir evenly, and cool to room temperature, the system turns into a solid paste, which is aseptically packaged to obtain the product.
[0029] Effect experiment 1 verification of the properties of the product of the present invention
[0030] The product obtained by the present invention is a yellow paste solid at normal temperature, has strong wall adhesion, and does not fall off the wall after being inve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com